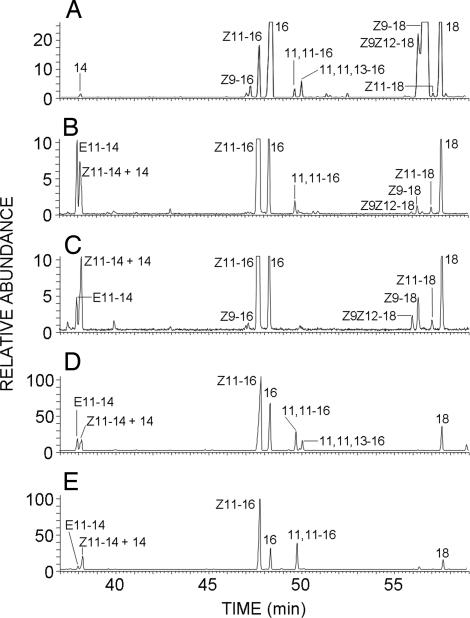
Fig. 5.
GC/MS analysis of lipid extracts from yeast transformed with desaturase genes. Methanolyzed lipid extracts from T. pytiocampa pheromone glands (A) and yeast transformed with either pYEXTHS-BN-Tpi-PGFAD (B and D) or pYEXTHS-BN-SlsZ/E11 (S. littoralis pheromone gland Δ11/Δ10,12 desaturase) (C and E) were analyzed. Traces correspond to the ion current obtained by extraction of the molecular ions of methyl tetradecanoate (m/z 242), methyl tetradecenoates (m/z 240), methyl tetradecadienoates (m/z 238), methyl hexadecanoate (m/z 270), methyl hexadecenoates (m/z 268), methyl hexadecadienoates (m/z 266), methyl octadecanoate (m/z 298), methyl octadecenoates (m/z 298), methyl octadecadienoates (m/z 296), and the diagnostic ions of methyl 11-hexadecynoate (m/z 96) and methyl (Z)-13-hexadecen-11-ynoate (m/z 94). The mass spectra of compounds labeled as 11,11–16 in trace B (retention time 49.7 min) and 11,11,13–16 in trace D (retention time 50.0 min) are shown in Fig. 6 C and E, respectively, and those of their corresponding synthetic standards are depicted in Fig. 6 D and F. Transformants were grown in the presence of 2 mM CuSO4 with (D and E) or without (B and C) 0.5 mM 11-hexadecynoic acid. 14, methyl tetradecanoate; E11–14, methyl (E)-11-tetradecenoate; Z11–14, methyl (Z)-11-tetradecenoate; 16, methyl hexadecanoate; Z9–16, methyl (Z)–9–hexadecenoate; Z11–16, methyl (Z)–11–hexadecenoate; 11,11–16, methyl 11-hexadecynoate; 11,11,13–16:Me, methyl (Z)-13-hexadecen-11-ynoate; 18, methyl octadecanoate; Z9–18, methyl (Z)–9–octadecenoate; Z11–18, methyl (Z)–11–octadecenoate; Z9Z12–18, methyl (Z,Z)-9,12-octadecadienoate.