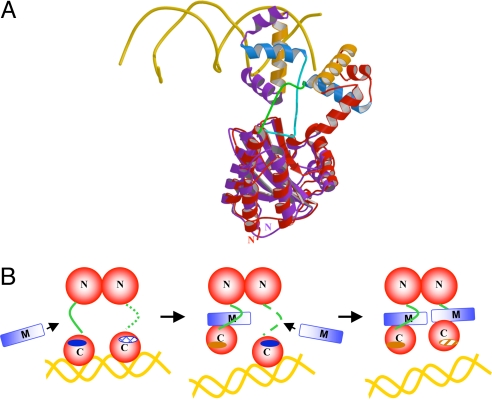
Fig. 4.
Mechanism of TraM inhibition of TraR. (A) Comparison of TraRAt–DNA with TraRNGR–TraMNGR structures. The NTDs of the both structures are superimposed, but for clarity, only one protomer of TraRNGR (in red) and TraRAt (in purple) from each structure is shown. α10, the TraR helix primarily responsible for TraM interactions, is light blue, and α12, the DNA binding helix, is orange. DNA is displayed as a double coil and is gold. The linker of TraRNGR is highlighted in green, and that of TraRAt is in cyan. The image was generated by using MOLSCRIPT and RASTER 3D (28, 29). (B) The proposed stepwise dissociation of TraRNGR–DNA by TraMNGR. One of the linkers in TraR–DNA, disordered and thus not observed crystallographically, is represented by the dotted line. The exposed TraM-binding site is indicated by a dark blue solid oval, and the buried site is denoted by a hatch-filled oval. The DNA-binding site facing the reader is represented as a dark orange solid oval, and that facing away from the reader as a shaded oval.