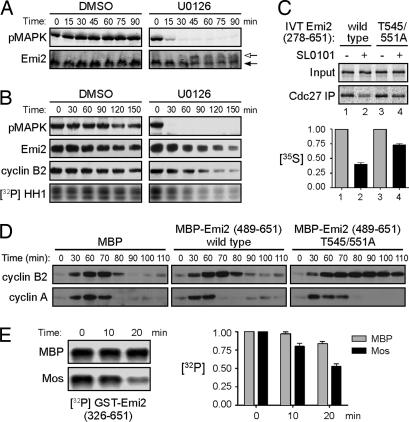
Fig. 2.
Mos regulates Emi2 T545/551 dephosphorylation. (A) CSF extracts were incubated at room temperature in the presence of DMSO or 200 μM U0126. Samples were taken at the indicated times and were immunoblotted for pMAPK and Emi2. Arrows indicate Emi2 mobility shift. (B) CSF extracts were incubated at room temperature in the presence of DMSO or 250 μM U0126. At the indicated times, samples were withdrawn to examine pMAPK and cyclin B2. Samples were also taken to examine endogenous Emi2 after λ-phosphatase treatment and to measure Cdc2/cyclin B kinase activity. (C) (Upper) CSF extracts were supplemented with 40 nM nondegradable cyclin B1. After 90 min, IVT 35S-labeled Emi2 was added in the presence or absence of Rsk inhibitor, SL0101. After 30 min of incubation, Cdc27 was immunoprecipitated and washed, and bound Emi2 was examined by SDS/PAGE and autoradiography. (Lower) The amount of 35S-labeled Emi2 bound to Cdc27 was quantified, normalized, and plotted. Error bars represent the standard deviation of three replicates. (D) Cycling extracts were incubated at room temperature in the presence of MBP, MBP-Emi2 (489–651) WT, or MBP-Emi2 (489–651) T545/551A proteins. Samples were taken at indicated times, and levels of cyclins A and B2 were examined by immunoblotting. (E) (Left) GST-Emi2 (326–651) bound to glutathione Sepharose was phosphorylated in vitro with Cdc2/cyclin B and [γ-32P]ATP and then incubated in interphase extracts pretreated with MBP or MBP-Mos. At indicated times, beads were washed, and the remaining phospho-Emi2 was examined by SDS/PAGE and autoradiography. (Right) The amount of 32P-labeled Emi2 remaining was quantified, normalized, and plotted. Each column represents the average of three replicates, and error bars represent the standard deviation.