Figure 1.
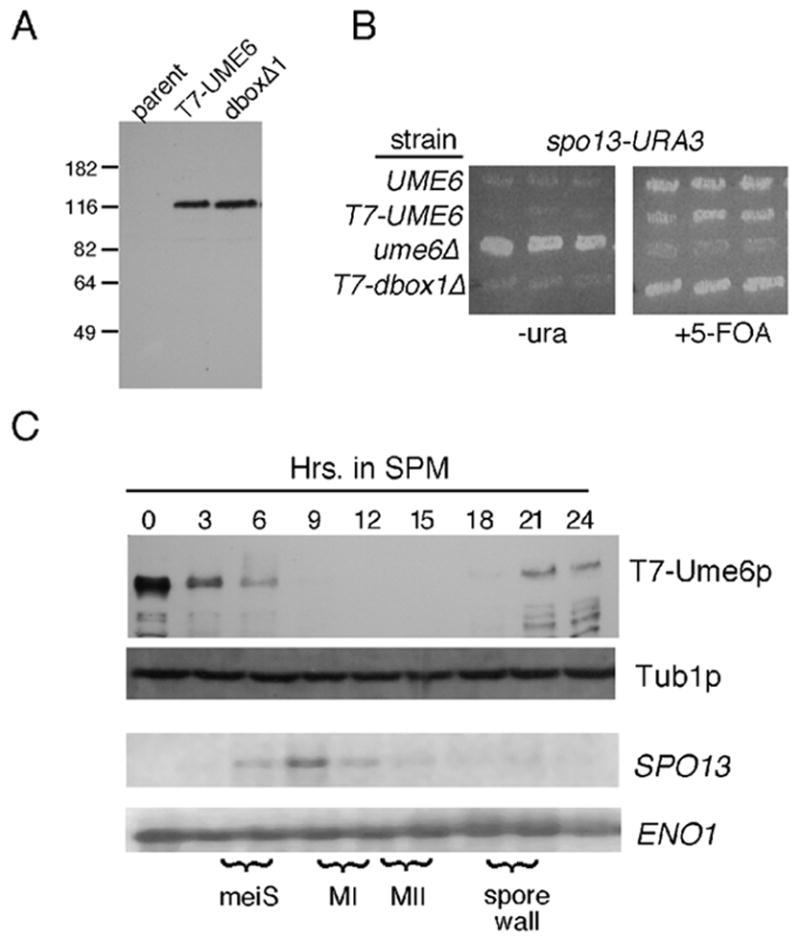
Ume6p regulation during meiosis. Panel A. Vegetative steady state levels of T7-epitope tagged wild-type strain RSY1079 (T7-Ume6p) and destruction box 1 mutant (RSY1180, Ume6pdb1Δ) proteins were determined Western blot analysis. The parental control (RSY335) lacks a T7-tagged UME6 allele. Molecular weight standards (kDa) are given on the left of the panel. Panel B. The indicated strains UME6 (RSY335), T7-UME6 (RSY1079), ume6Δ (RSY853) and T7-dbox1Δ (RSY1080) were transformed with the spo13-URA3 reporter plasmid (pMS49). Three independent transformants were patched on medium lacking uracil (-uracil) or containing the uracil analog 5-FOA. Plates were incubated two days and photographed. Panel C. A meiotic timecourse was conducted with strain RSY1079 and timepoints taken prior (0 hr) and following the shift to sporulation medium (SPM). T7-Ume6p levels were determined by Western blot analysis. Northern blot analysis of SPO13 mRNA was conducted from the same timepoints. The execution of meiotic S phase (meiS), the meiotic divisions (MI, MII) and spore wall formation were determined by FACS analysis, fluorescent microscopy of DAPI stained cells and light microscopy, respectively. Tub1p and ENO1 mRNA levels served as loading controls for the Western and Northern blot analyses, respectively.
