Figure 3.
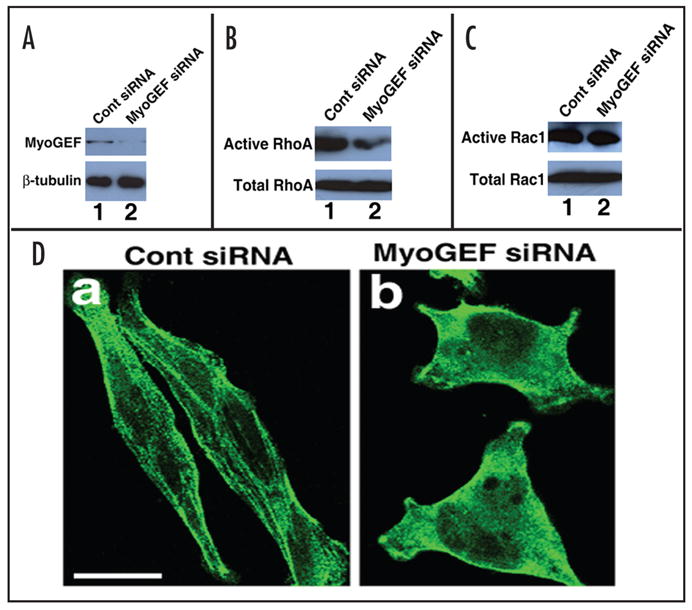
MyoGEF activates RhoA that in turn induces myosin filament formation. (A) Downregulation of MyoGEF protein in HeLa cells by RNA interference (RNAi). HeLa cells were transfected with control siRNA (lane1) or MyoGEF siRNA (lane 2). Foutry-eight hours after transfection, the transfected HeLa cells were subjected to an immunoblot analysis using antibodies specific for MyoGEF (upper panel) and beta-tubulin (lower panel). Note that MyoGEF siRNA significantly reduces MyoGEF (but not beta-tubulin) protein level (compare lane 1 with lane 2). (B) MyoGEF activates RhoA. The transfected HeLa cells in (A) were subjected to a specific RhoA activation assay. Note that inactivation of MyoGEF in Hela cells significantly reduces the active RhoA level (compare lane 1 with lane 2). (C) MyoGEF does not activate Rac1. The transfected HeLa cells in (A) were subjected to a specific Rac activation assay. Note that inactivation of MyoGEF in Hela cells does not affect the active Rac1 level (compare lane 1 with lane 2). (D) MyoGEF is important for myosin filament formation. The transfected HeLa cells in (A) were fixed and stained with an antibody specific for non-muscle myosin II-A. Note that inactivation of MyoGEF in HeLa cells significantly reduces myosin filaments. Bar, 10 μm.
