Figure 1.
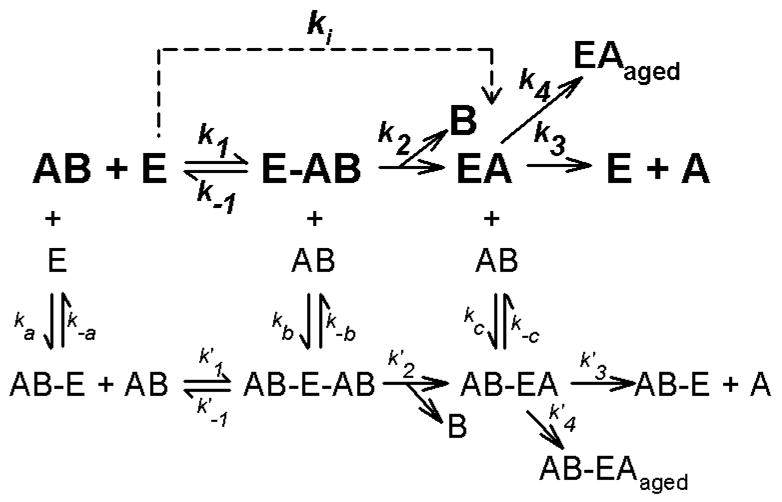
Kinetic schemes descriptive of the interactions of acetylthiocholine, acetylcholine, or organophosphorus insecticide oxons with acetylcholinesterase. In this scheme, E represents free acetylcholinesterase, while AB represents substrate or inhibitor. Ligand bound to a secondary site is indicated by AB–E, whereas the Michaelis complex is denoted by E-AB. EA represents acetylated or phosphorylated enzyme, and B depicts the leaving groups. EAaged designates aged, enzyme-inhibitor complex. The pathway in bold depicts the simplest kinetic scheme, utilized by Main (1964), where binding only occurs at the active site. Aging and reactivation are often ignored when k3 and k4 are small. The ki represents k2/Kd under pseudo first order conditions (Main, 1964). The additional, non-bold pathways show a much more complex kinetic scheme that includes reversible binding to a secondary site. In the case of acetylthiocholine (and acetylcholine) this secondary site is the peripheral anionic site. In the case of organophosphates no such secondary site has been identified, but is suggested on the basis of kinetic data.
