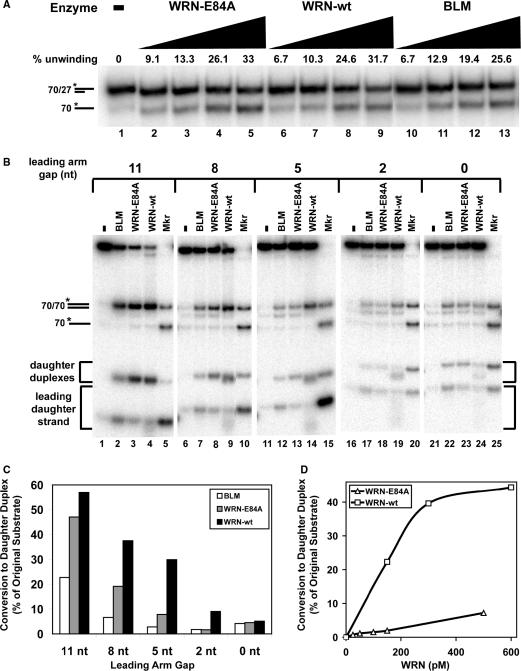
Figure 2.
Wild-type WRN (WRN-wt) catalyzes more efficient regression of fork substrates with shorter leading arm gaps than exonuclease-deficient WRN-E84A or BLM. (A) Unwinding activities of WRN-E84A (60, 120, 360, 480 pM), WRN-wt (60, 120, 360, 480 pM), or BLM (60, 120, 180, 240 pM) on partial duplex substrate (*70lag/27lag) over 5 min at 37°C were compared. Duplex and single-stranded DNA products were separated by non-denaturing PAGE and quantitated after phophorimaging, with amounts of enzyme-mediated unwinding presented above each lane. (B) Reactions containing fork substrates (50 pM) with leading strand gaps of 11 nt (lanes 1–4), 8 nt (lanes 6–9), 5 nt (lanes 11–14), 2 nt (lanes 16–19) or 0 nt (lanes 21–24) without or with BLM (160 pM), WRN-E84A (120 pM) or WRN-wt (120 pM) as indicated were incubated at 37°C for 5 min. DNA products were analyzed by native PAGE and visualized by phosphorimaging. Also subject to PAGE in parallel were marker sample mixtures (asterisks below denoting the radiolabeled strands) each containing parental duplex (*70lag/70lead) and single-stranded 70-mer (*70lag) markers but distinguished by substrate-specific daughter duplex and leading daughter strand markers as follows: *21lead/30lag and *21lead (lane 5), *24lead/30lag and *24lead (lane 10), *27lead/30lag and *27lead (lane 15), *30lead/30lag and *30lead (lane 20), and *32lead/30lag and *32lead (lane 25). (C) For the reactions analyzed in B containing various fork substrates and BLM, WRN-E84A or WRN-wt, the percentage of daughter duplex formation relative to the original (molar) amount of intact fork substrate is quantitated as described in ‘Materials and methods’ section. This data is plotted in bar graph form showing the relative efficiencies of BLM (white), WRN-E84A (gray) and WRN-wt (black) in forming daughter duplex from replication fork substrates with leading strand gaps of 11, 8, 5, 2 and 0 nt. (D) Regression reactions containing fork substrate (50 pM) with a leading strand gap of 5 nt and either WRN-wt (150, 300 or 600 pM) or WRN-E84A (25, 50, 100, 150 or 500 pM) were incubated 5 min at 37°C and analyzed as described in B. The molar amount of daughter duplex (with respect to the amount of fork substrate) generated at each WRN-wt (squares) or WRN-E84A (triangles) concentration is plotted.