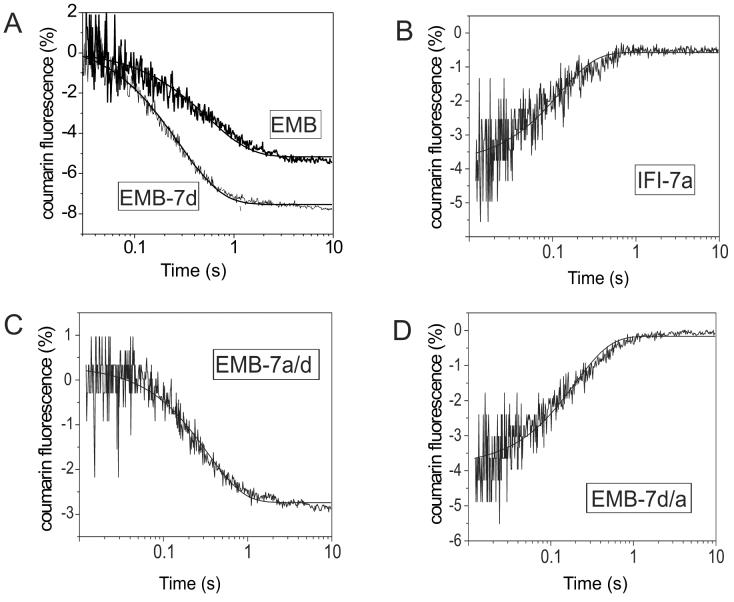
Figure 3.
Rate of eda-deac ADP dissociation (k−D) from Drosophila S1 isoforms
The rate constant for ADP dissociation (k−D) from S1 in the absence of actin was determined. Addition of ATP (15 μM) from cagedATP (100 μM) by a single laser flash displaced the eda-deac ADP bound to S1. The change in eda-deac ADP fluorescence upon release from S1 was measured and fit with a single exponential to determine k−D. Exchange of either the exon 7a or 7d domain resulted in a modulation of the eda-deac ADP release rate. The dissociation rate for EMB-7d (A: 4.3 s−1) is faster than EMB (A: 1.8 s−1) while that of IFI-7a (B: 4.7 s−1) is slower than IFI (7.5 s−1). The observed rate constant of eda-deac ADP dissociation (k−D) from EMB-7a/7d (C) and EMB-7d/7a (D) S1 gave mean values of 6.1 s−1 and 2.6 s−1, respectively. The conditions were: 20 mM MOPS, 30 mM KCl, 5 mM MgCl2, 10 mM DTT, pH 7.