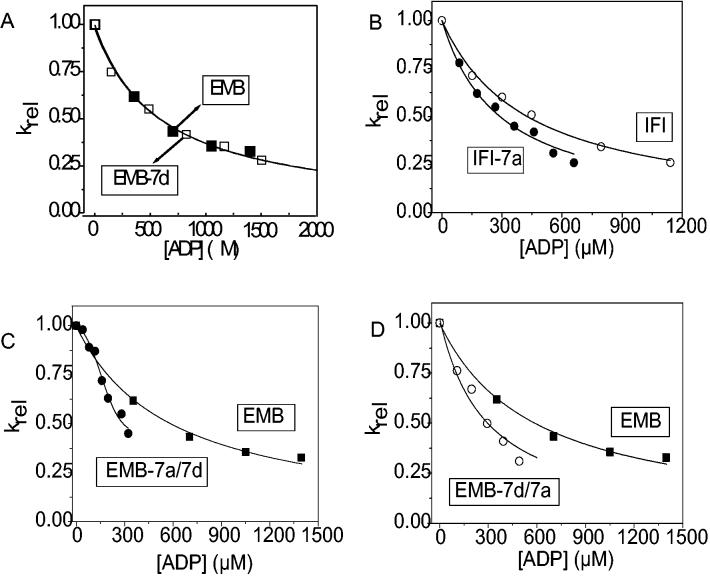
Figure 5.
The affinity of ADP (KAD) for acto-S1
The dissociation of acto-S1 was induced by ATP in the presence of ADP ranging from 0-1500 μM. The light scattering traces were fitted with single exponentials to determine the kobs. Hyperbolic plots of the kobs vs. ADP concentration were fitted with an equation derived from Scheme 1 (kobs = K1k+2([ATP]/(1 + [ADP]/KAD)) to determine KAD. Shown are the relative kobs (krel) vs. ADP concentration plots for easier comparison. A) The fits yielded a value of 607 ± 44 μM for EMB-7d (empty squares) as compared to 587 ± 38 μM for EMB (filled squares). B) The fits yielded values of 409 ± 26 μM (open circles) for IFI and 239 ± 32 μM for IFI-7a (closed circles). The KAD for EMB-7a/7d (C) and EMB-7d/7a (D) S1 isoforms were measured with the following alterations. The dissociation of acto-S1 was calculated in the presence of 0 – 400 μM ADP and the KAD for EMB-7a/7d S1 was determined to be the ADP concentration at 50% inhibition. All preparations for EMB-7a/7d produced the sigmoidal-shaped curve observed here. The fits yielded values of 191 ± 59 μM for EMB-7a/7d and 220 ± 33 μM for EMB-7d/7a.