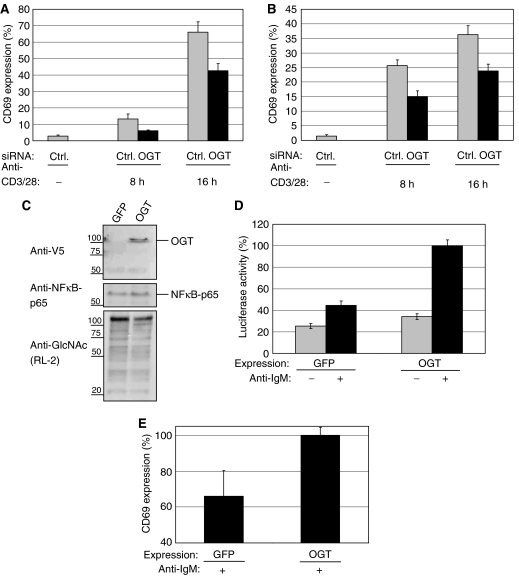
Figure 2.
OGT knockdown impairs, whereas overexpression sensitizes T-cell and B-cell toward early activation. (A) Jurkat cells were transfected either with a negative control siRNA (Ctrl) or siRNA specific for OGT (OGT_1). After transfection, the cells were transferred to fresh culture medium for 24 h before stimulation with plate-bound anti-CD3/28 antibodies for 8 or 16 h, respectively. After stimulation, CD69 surface expression was measured by Flow cytometry as described in Materials and methods. Experiments were performed in triplicates. CD69 externalization after OGT or Ctrl transfection is given as a percentage of the maximal externalization obtained by treating with PMA/ionomycin, set as 100%. (B) BJAB cells were either transfected with a negative Ctrl or siRNA specific for OGT (OGT_1). After transfection, the cells were transferred to fresh culture medium for 24 h before stimulation with anti-IgM antibodies for 8 or 16 h, respectively. After stimulation, CD69 surface expression was measured as in (A). (C) BJAB cells were transfected with plasmids encoding GFP as a control or OGT and transferred to fresh medium for 48 h. With an antibody to the V5 tag, total cellular lysates were analyzed for expression of OGT. NFκB-p65 was blotted as loading control. The overall amount of O-GlcNAc levels were blotted with antibodies to O-GlcNAc (RL-2). (D) BJAB cells were co-transfected with a luciferase reporter plasmid encoding a NFκB reporter together with either GFP (as a control) or full-length OGT. Cells were incubated for 24 h and stimulated with anti-IgM antibodies for additional 12 h (+) or left unstimulated (−). After lysis, the luciferase activity was measured as described in Materials and methods. Experiments were performed in triplicates. The maximal NFκB activity observed after OGT overexpression and anti-IgM stimulation was set to 100%. (E) BJAB cells were transfected with plasmids for the overexpression of GFP (as a control) or of the full-length OGT. Cells were transferred to fresh medium for 48 h and stimulated with anti-IgM antibodies for 12 h. The maximal CD69 externalization observed after OGT overexpression and anti-IgM stimulation was set to 100%.