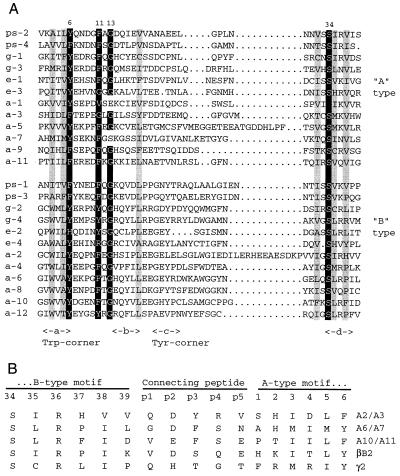
Figure 3.
(A) Alignment of the 12 βγ motifs of AIM1 (a1-a12) with A- and B-type βγ motifs from Protein S (ps-1 through ps-4), bovine γB-crystallin (g-1 through g-4), and EDSP (e-1 through e-4) (7). Numbers above indicate the positions of structurally critical residues in motif 1 of bovine γB-crystallin. The positions of β-strands a, b, c, and d are indicated below, along with the characteristic Trp and Tyr corners of B-type motifs. Important residues highlighted in black are glycine at position 13, aromatic residues at positions 6 and 11, and serine at position 34. Residues highlighted in gray are generally hydrophobic and contribute to packing between β-sheets. (B) Alignment of AIM1-connecting peptide sequences (a2/a3, a6/a7, and a10/a11) with those of bovine βB2- and γB-crystallin. For comparison, motif sequences are numbered according to the scheme for motif 1 of γB. The extra residues of the connecting peptide are numbered p1–p5.