Abstract
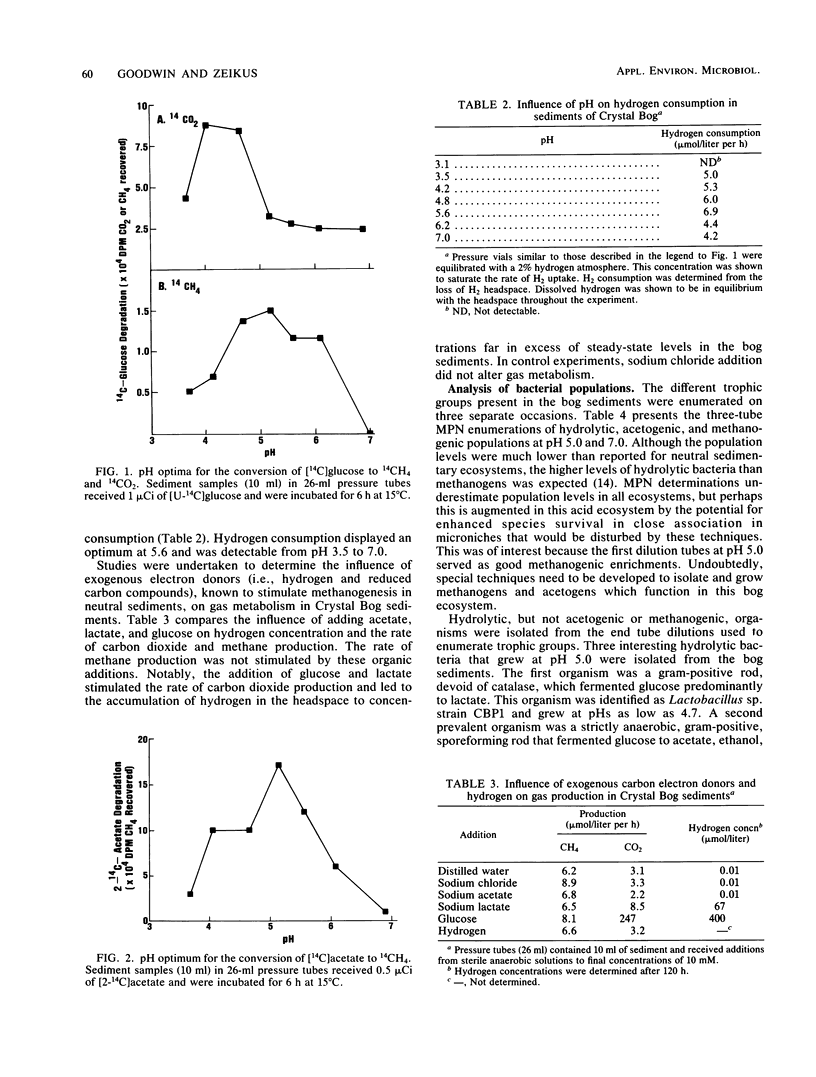
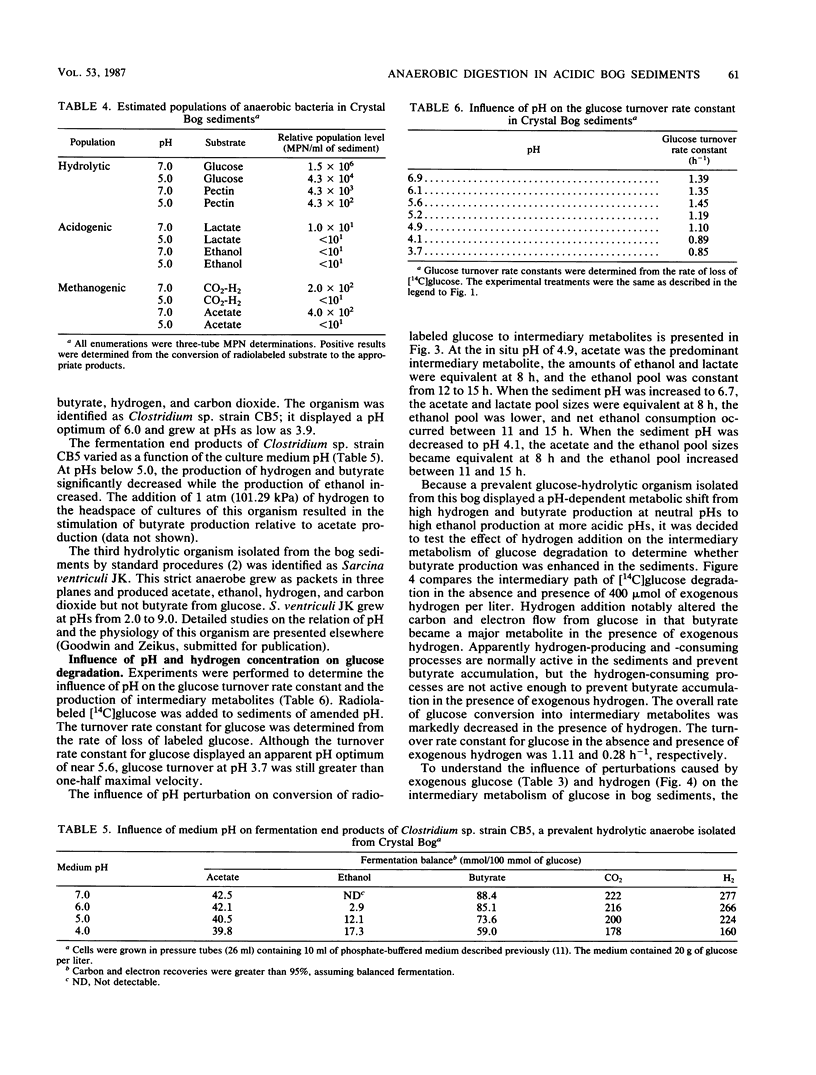
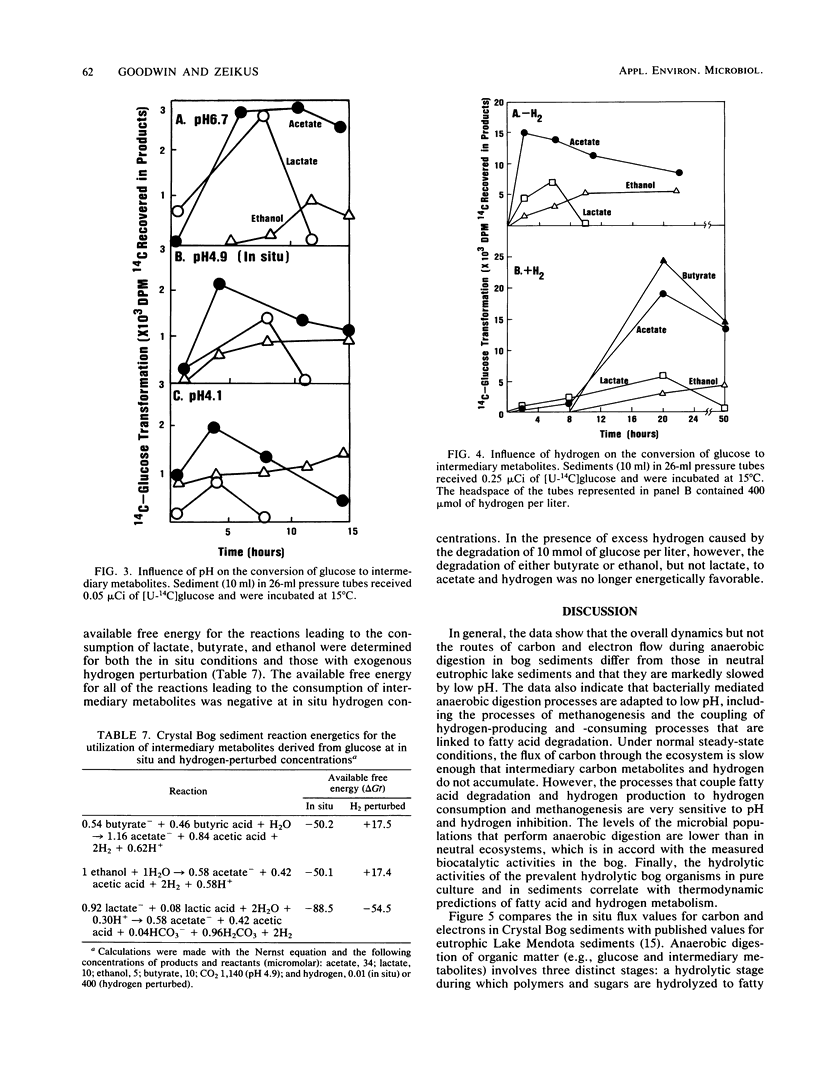
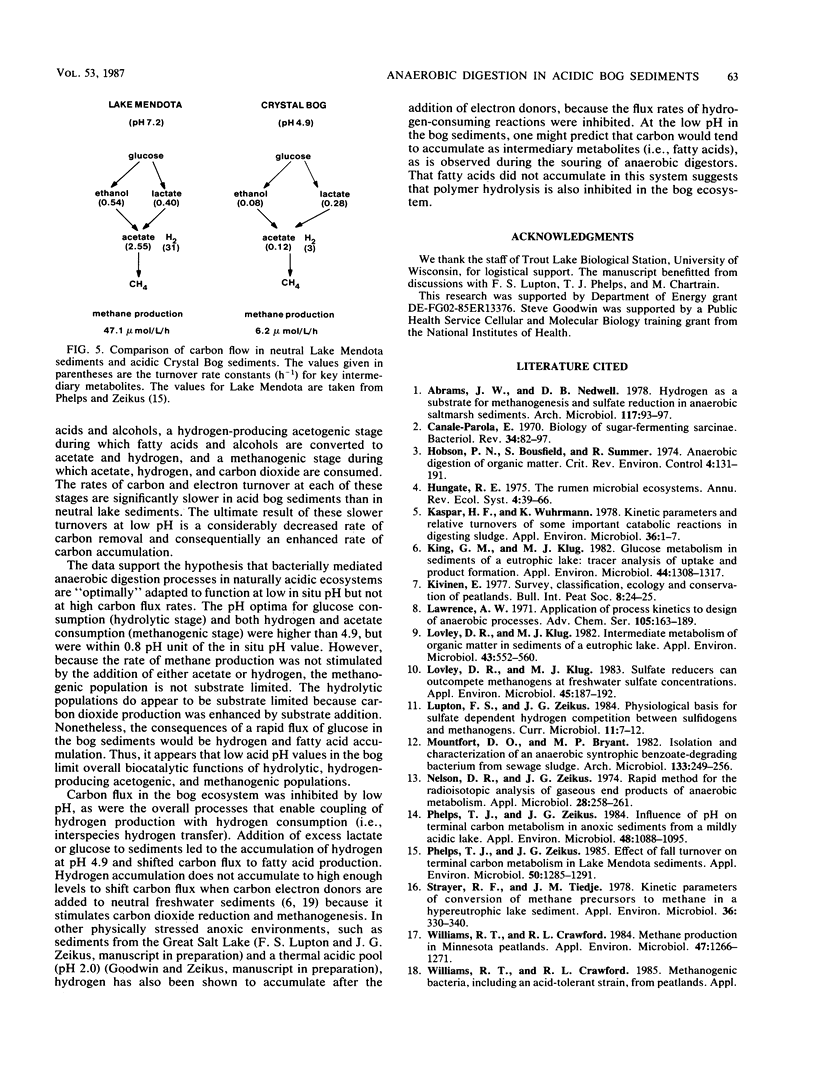
The dynamics of anaerobic digestion were examined in the low-pH sediments of Crystal Bog in Wisconsin. The sediments (pH 4.9) contained 71% organic matter and the following concentrations of dissolved gases (micromoles per liter): CO2, 1,140; CH4, 490; and H2, 0.01. The rate of methane production was 6.2 mumol/liter of sediment per h, which is slower than eutrophic, neutral sediments. Microbial metabolic processes displayed the following pH optima: hydrolysis reactions, between 4.2 and 5.6; aceticlastic methanogenesis, 5.2; and hydrogen-consuming reactions, 5.6. The turnover rate constants for key intermediary metabolites were (h-1): glucose, 1.10; lactate, 0.277; acetate, 0.118; and ethanol, 0.089. The populations of anaerobes were low, with hydrolytic groups (10(6)/ml) several orders of magnitude higher than methanogens (10(2)/ml). The addition of carbon electron donors to the sediment resulted in the accumulation of hydrogen, whereas the addition of hydrogen resulted in the accumulation of fatty acids and the inhibition of hydrogen-producing acetogenic reactions. Strains of Lactobacillus, Clostridium, and Sarcina ventriculi were isolated from the bog, and their physiological attributes were characterized in relation to hydrolytic process functions in the sediments. The present studies provide evidence that the pH present in the bog sediments alter anaerobic digestion processes so that total biocatalytic activity is lower but the general carbon and electron flow pathways are similar to those of neutral anoxic sediments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram J. W., Nedwell D. B. Hydrogen as a substrate for methanogenesis and sulphate reduction in anaerobic saltmarsh sediment. Arch Microbiol. 1978 Apr 27;117(1):93–97. doi: 10.1007/BF00689357. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E. Biology of the sugar-fermenting Sarcinae. Bacteriol Rev. 1970 Mar;34(1):82–97. doi: 10.1128/br.34.1.82-97.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Wuhrmann K. Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl Environ Microbiol. 1978 Jul;36(1):1–7. doi: 10.1128/aem.36.1.1-7.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M., Klug M. J. Glucose metabolism in sediments of a eutrophic lake: tracer analysis of uptake and product formation. Appl Environ Microbiol. 1982 Dec;44(6):1308–1317. doi: 10.1128/aem.44.6.1308-1317.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Sulfate reducers can outcompete methanogens at freshwater sulfate concentrations. Appl Environ Microbiol. 1983 Jan;45(1):187–192. doi: 10.1128/aem.45.1.187-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps T. J., Zeikus J. G. Effect of fall turnover on terminal carbon metabolism in lake mendota sediments. Appl Environ Microbiol. 1985 Nov;50(5):1285–1291. doi: 10.1128/aem.50.5.1285-1291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps T. J., Zeikus J. G. Influence of pH on Terminal Carbon Metabolism in Anoxic Sediments from a Mildly Acidic Lake. Appl Environ Microbiol. 1984 Dec;48(6):1088–1095. doi: 10.1128/aem.48.6.1088-1095.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer R. F., Tiedje J. M. Kinetic parameters of the conversion of methane precursors to methane in a hypereutrophic lake sediment. Appl Environ Microbiol. 1978 Aug;36(2):330–340. doi: 10.1128/aem.36.2.330-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. T., Crawford R. L. Methane production in Minnesota peatlands. Appl Environ Microbiol. 1984 Jun;47(6):1266–1271. doi: 10.1128/aem.47.6.1266-1271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Microbial methanogenesis and acetate metabolism in a meromictic lake. Appl Environ Microbiol. 1979 Feb;37(2):213–221. doi: 10.1128/aem.37.2.213-221.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Winfrey M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl Environ Microbiol. 1976 Jan;31(1):99–107. doi: 10.1128/aem.31.1.99-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


