Abstract
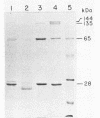
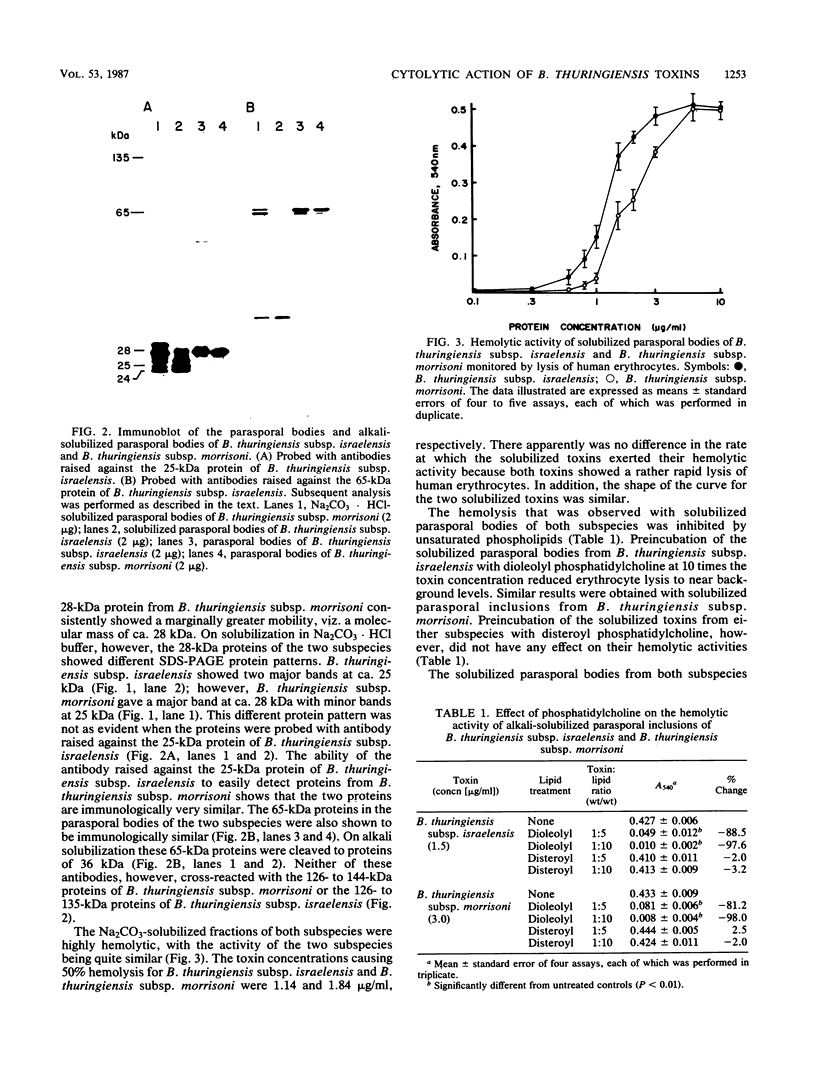
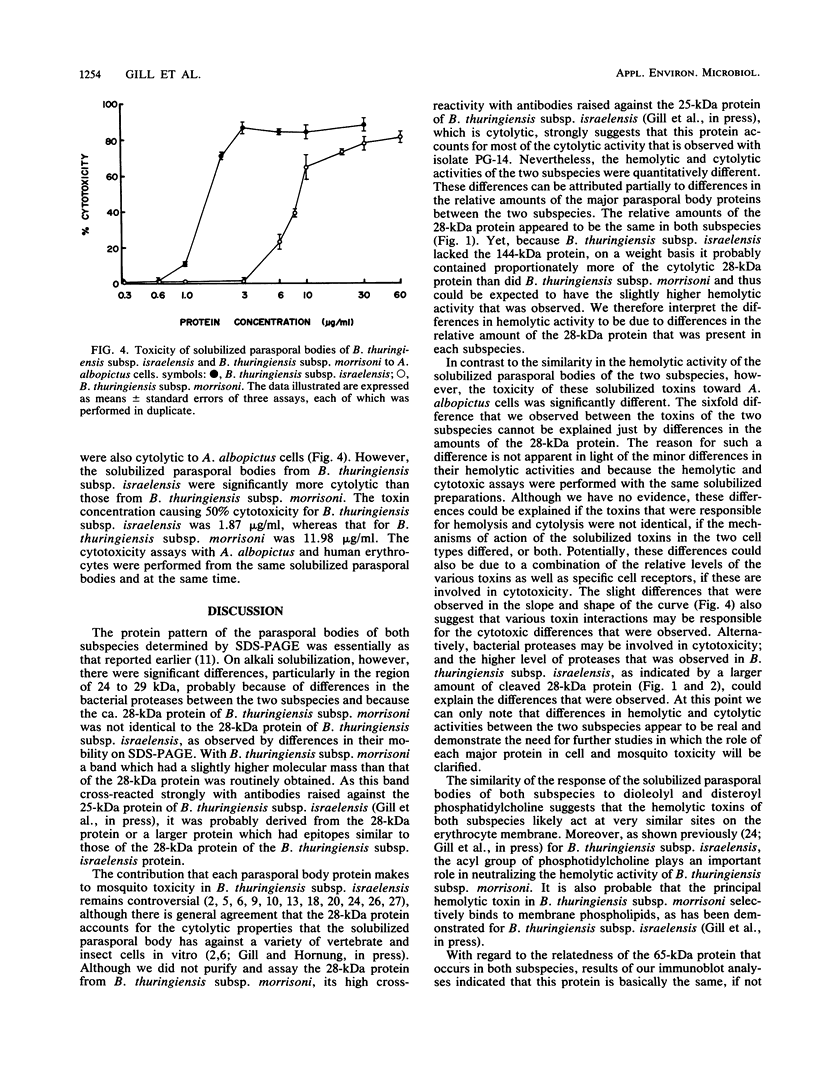
The parasporal bodies of the mosquitocidal isolates of Bacillus thuringiensis subsp. israelensis and B. thuringiensis subsp. morrisoni isolate PG-14 were compared with regard to their hemolytic and cytolytic activities and the immunological relatedness of the 28- and 65-kilodalton (kDa) proteins that occur in both subspecies. The alkali-solubilized parasporal bodies of B. thuringiensis subsp. israelensis caused 50% lysis of human erythrocytes at 1.14 micrograms/ml, whereas those of B. thuringiensis subsp. morrisoni caused similar lysis at 1.84 micrograms/ml. Preincubation of solubilized parasporal bodies with dioleolyl phosphatidylcholine significantly inhibited the hemolytic activity of both supspecies. In cytolytic assays against Aedes albopictus cells, the toxin concentrations causing 50% lysis for B. thuringiensis subsp. israelensis and B. thuringiensis subsp. morrisoni were 1.87 and 11.98 micrograms/ml, respectively. Polyclonal antibodies raised separately against the 25-kDa protein (a tryptic digest of the 28-kDa protein) or the 65-kDa protein of B. thuringiensis subsp. israelensis cross-reacted, respectively, with the 28- and the 65-kDa proteins of B. thuringiensis subsp. morrisoni. However, neither of these antibodies cross-reacted with the 135-kDa protein of either subspecies. These results indicate that the mosquitocidal and hemolytic properties of B. thuringiensis subsp. israelensis and B. thuringiensis subsp. morrisoni isolate PG-14 are probably due to the biologically related proteins that are present in the parasporal bodies of both subspecies. The lower hemolytic activity of the B. thuringiensis subsp. morrisoni may be due to the presence of lower levels of the 28-kDa protein in that subspecies.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang B. J., Nickerson K. W. Purification of the protein crystal from Bacillus thuringiensis by zonal gradient centrifugation. Appl Environ Microbiol. 1978 Oct;36(4):625–626. doi: 10.1128/aem.36.4.625-626.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. L., Rohrmann G. F., Beaudreau G. S. Delta endotoxin of Bacillus thuringiensis subsp. israelensis. J Bacteriol. 1985 Jan;161(1):39–46. doi: 10.1128/jb.161.1.39-46.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Charles J. F., de Barjac H. Action des cristaux de Bacillus thuringiensis var. israelensis sur l'intestin moyen des larves de Aedes aegypti L., en microscopie électronique. Ann Microbiol (Paris) 1983 Mar-Apr;134A(2):197–218. [PubMed] [Google Scholar]
- Hurley J. M., Lee S. G., Andrews R. E., Jr, Klowden M. J., Bulla L. A., Jr Separation of the cytolytic and mosquitocidal proteins of Bacillus thuringiensis subsp. israelensis. Biochem Biophys Res Commun. 1985 Jan 31;126(2):961–965. doi: 10.1016/0006-291x(85)90279-7. [DOI] [PubMed] [Google Scholar]
- Ibarra J. E., Federici B. A. Isolation of a relatively nontoxic 65-kilodalton protein inclusion from the parasporal body of Bacillus thuringiensis subsp. israelensis. J Bacteriol. 1986 Feb;165(2):527–533. doi: 10.1128/jb.165.2.527-533.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Eckblad W., Bulla L. A., Jr Diversity of protein inclusion bodies and identification of mosquitocidal protein in Bacillus thuringiensis subsp. israelensis. Biochem Biophys Res Commun. 1985 Jan 31;126(2):953–960. doi: 10.1016/0006-291x(85)90278-5. [DOI] [PubMed] [Google Scholar]
- Padua L. E., Ohba M., Aizawa K. The isolates of Bacillus thuringiensis serotype 10 with a highly preferential toxicity to mosquito larvae. J Invertebr Pathol. 1980 Sep;36(2):180–186. doi: 10.1016/0022-2011(80)90022-1. [DOI] [PubMed] [Google Scholar]
- Pfannenstiel M. A., Couche G. A., Ross E. J., Nickerson K. W. Immunological relationships among proteins making up the Bacillus thuringiensis subsp. israelensis crystalline toxin. Appl Environ Microbiol. 1986 Oct;52(4):644–649. doi: 10.1128/aem.52.4.644-649.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar V. Biochemical and immunological characterization of the cloned crystal toxin of Bacillus thuringiensis var. israelensis. Biochem Biophys Res Commun. 1986 Jun 13;137(2):748–751. doi: 10.1016/0006-291x(86)91142-3. [DOI] [PubMed] [Google Scholar]
- Singh G. J., Schouest L. P., Jr, Gill S. S. Action of Bacillus thuringiensis subsp. israelensis delta-endotoxin on the ultrastructure of the house fly larva neuromuscular system in vitro. J Invertebr Pathol. 1986 Mar;47(2):155–166. doi: 10.1016/0022-2011(86)90042-x. [DOI] [PubMed] [Google Scholar]
- Thomas W. E., Ellar D. J. Bacillus thuringiensis var israelensis crystal delta-endotoxin: effects on insect and mammalian cells in vitro and in vivo. J Cell Sci. 1983 Mar;60:181–197. doi: 10.1242/jcs.60.1.181. [DOI] [PubMed] [Google Scholar]
- Thomas W. E., Ellar D. J. Mechanism of action of Bacillus thuringiensis var israelensis insecticidal delta-endotoxin. FEBS Lett. 1983 Apr 18;154(2):362–368. doi: 10.1016/0014-5793(83)80183-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]