Abstract
PURPOSE
Psychological interventions are efficacious in reducing emotional distress for cancer patients. However, it is not clear whether psychological improvements are, in turn, related to improved health. A clinical trial tests whether a psychological intervention for cancer patients can do so, and also tests two routes to achieve better health: a) reducing patients’ emotional distress, and/or b) enhancing their functional immunity.
METHODS
Post-surgery, 227 breast cancer patients were randomized to Intervention or Assessment only study arms. Conducted in small groups, intervention sessions were offered weekly for 4 months and followed by monthly sessions for 8 months. Measures included psychological (distress), biological (immune), and health outcomes (performance status and evaluations of patient’s symptomatology, including toxicity from cancer treatment, lab values) collected at baseline, 4 months, and 12 months.
RESULTS
A path model revealed that intervention participation directly improved health (p<.05) at 12 months. These effects remained when statistically controlling for baseline levels of distress, immunity, and health as well as sociodemographic, disease, and cancer treatment variables. Regarding the mechanisms for achieving better health, support was found for an indirect effect of distress reduction. That is, by specifically lowering intervention patients’ distress at 4 months, their health was improved at 12 months (p<.05). Although the intervention simultaneously improved patients’ T-cell blastogenesis in response to phytohemagglutinin (PHA), the latter increases were unrelated to improved health.
CONCLUSION
A convergence of biobehavioral effects and health improvements were observed. Behavioral change, rather than immunity change, was influential in achieving lower levels of symptomatology and higher functional status. Distress reduction is highlighted as an important mechanism by which health can be improved.
Keywords: psychological, health, immune, quality of life, breast cancer, biobehavioral
INTRODUCTION
Stress is implicated in the development of physical symptoms such as pain and fatigue, as well as in reductions in physical quality of life for cancer patients (Spector and Jex, 1998; Golden-Kreutz et al., 2005). Psychological interventions are a plausible strategy to modify this scenario as they have shown substantial efficacy in reducing emotional distress for cancer patients (Dodd, 1988; Scheier et al., 2005; Goodwin et al., 2001; Yates et al., 2005). But are these gains accompanied by improved health for cancer patients?
There is a dearth of empirical tests, as the majority of randomized clinical trials (RCT) focus on emotional distress or quality of life outcomes rather than health (Andersen, 1992, 2002). Also, the findings are mixed regardless of the definition of ‘health’ used. It is infrequent to rate or measure health directly, but instead patients might provide ratings of the disruptive effects of symptoms on their daily lives. Measures such as the physical health component score of the SF-36 (Ware, Snow & Kosinski, 2000) or similar scales [European Organization for Research and Treatment of Cancer QLQ-30 (EORTC; Aaronson et al., 1993); Functional Assessment of Cancer Therapy Scale (FACT; Cella et al., 1993)] have been utilized. With such measures, a number of interventions have resulted in fewer disruptions in physical health-related quality of life (QoL; Edgar, Rosberger, & Collet, 2001; Helgeson et al., 1999) whereas others have not (Allen et al., 2002; de Wit et al., 1997; Edmonds et al. 1999; Giesler et al., 2005; Stanton et al., 2002). A more direct strategy has been patient ratings of a few specific symptoms, usually symptoms related to treatments received (e.g., a visual analogue scale used for rating nausea/vomiting with chemotherapy). In these studies, results have been generally favorable, with intervention patients self reporting significantly lower chemotherapy related symptoms (Roscoe, et al., 2000), fatigue (Yates et al., 2005; Quesnel et al., 2003), pain (de Wit et al., 1997; Doorenbos et al., 2006; Maguire et al., 1983; Spiegel & Bloom, 1983; Yates et al., 1981), and lymphedema (Maguire et al., 1983), as well as improvements across multiple symptoms (Forester et al., 1985; McCorkle et al., 1989). With the symptoms-focused studies, there have been fewer null findings [no differential improvement of urinary symptoms for prostate cancer patients (Giesler et al., 2005; Johnson et al., 1988); no improvement in lymphedema among breast cancer patients (Dodd, 1988; Stanton et al., 2002)].
Rather than self-evaluations, we were interested in testing the effects of an intervention on objective measures of health status typically used for oncology clinical trials. One global indicator used is the Karnofsky Performance Status Rating (Karnofsky & Burchenal, 1949), a broad evaluation of the patient’s mobility, symptomatology, and the resulting effects on current activity level and self care. As cancer treatment trials monitor patients closely to check for disease progression (rather than control), the tolerance for the next round of therapy, and the possible emergence of treatment toxicities or adverse events, there are multiple specific indicators used, such as findings from physical exams (e.g., blood pressure, neuromotor weakness), laboratory tests (e.g., liver functioning), or radiographs (e.g., chest films). Collaborative groups [(e.g., Gynecologic Oncology Group (GOG), the NSABP (National Surgical Adjuvant Breast and Bowel Project)] have combined these indicators into similar, standardized listings of symptoms, signs, illnesses, lab values, etc. used for their trials. In the listings, each item has a graded severity scale which is rated by nurses or physicians at frequent, protocol dependent intervals. In combination these two measures--functional status ratings and listings of symptoms, signs, etc.--document the health status of cancer treatment trial patients (e.g., Shapiro and Recht, 2001; Mouridsen et al., 2004; Pelegri et al., 2005) and are tested as correlates of disease endpoints (Mouridsen et al., 2003).
Our ongoing clinical trial tests whether a psychological intervention can alter the incidence of and/or time to recurrence for women with breast cancer. Although data are not yet available for an effect on disease endpoints, results are available for intermediate outcomes. Thus far, the intervention has shown robust biobehavioral effects (Andersen et al., 2004). Patients randomized to weekly, group intervention sessions for 4-months showed significant reductions in distress as well as significant health behavior changes, with patients high in initial cancer-related stress experiencing the greatest reduction in distress. Immunity was conceptualized as a potential mechanism linking lowered distress to health outcomes (Andersen et al., 1994). Analyses showed that T-cell proliferation in response to Phytohemagglutinin (PHA) and Concanavalin A (ConA) remained stable or increased for the Intervention group but declined for the Assessment only group (ps < .01). Because the intervention produced significant biobehavioral effects, we reasoned that improved health should, at some point, be evident at a later time point.
To increase the durability of the intervention effects, the 4-month intensive phase was followed by a maintenance phase of monthly sessions for an additional 8 months. At 12-months we reevaluated patients’ emotional distress and immunity, but relevant to the question and discussion above, we also assessed health status (Shapiro and Recht, 2001; Maltoni et al., 2005). We predicted that the intervention would have a direct, positive effect on health, operationalized with the indicators used in oncology trials.
In addition, we posed a second question: If improved health occurs, what is/are mechanism(s) for health change? As the focus of all cancer therapies is controlling the disease and thereby, improving health, it is important to identify how psychological interventions could achieve positive health effects. Psychological interventions commonly include many strategies that may affect multiple pathways (Dodd, 1988; Scheier et al., 2005). However, two pathways affecting health and/or disease processes have been frequently suggested, not only for cancer patients (Spiegel et al., 1989; Fawzy et al., 1993) but for other stressed populations as well (Kiecolt-Glaser et al., 2002). Namely, a psychological intervention might improve health by a) reducing emotional distress, and/or b) enhancing functional immunity. Thus, we test if a psychological intervention can directly improve the health of breast cancer patients, and also test two indirect pathways to improved health.
METHODS
Participants and Design
Patients newly diagnosed with regional breast cancer participated. Description of eligibility, procedures of accrual and randomization, and equivalence of study arms across sociodemographic, disease, and treatment variables have been detailed (Andersen et al., 1998; Andersen et al., 2004; see also Figure 1). Patients (N = 227) had been surgically treated with breast conserving therapy (43%) or mastectomy (57%) and were awaiting the start of adjuvant therapy. As previously described, the typical patient was 51 years old, Caucasian (90%), married (67%), had some college (72%), was employed at least part time (67%), and had a family income of more than $50 thousand/year (56%). The majority had Stage II (90%) rather than Stage III (10%) disease, were estrogen receptor positive (68%), and pre-menopausal (54%). The majority of patients also received adjuvant therapy and Table 1 details the percentages of patients in each study arm who received radiation and/or chemotherapy during the study year.
Fig. 1.
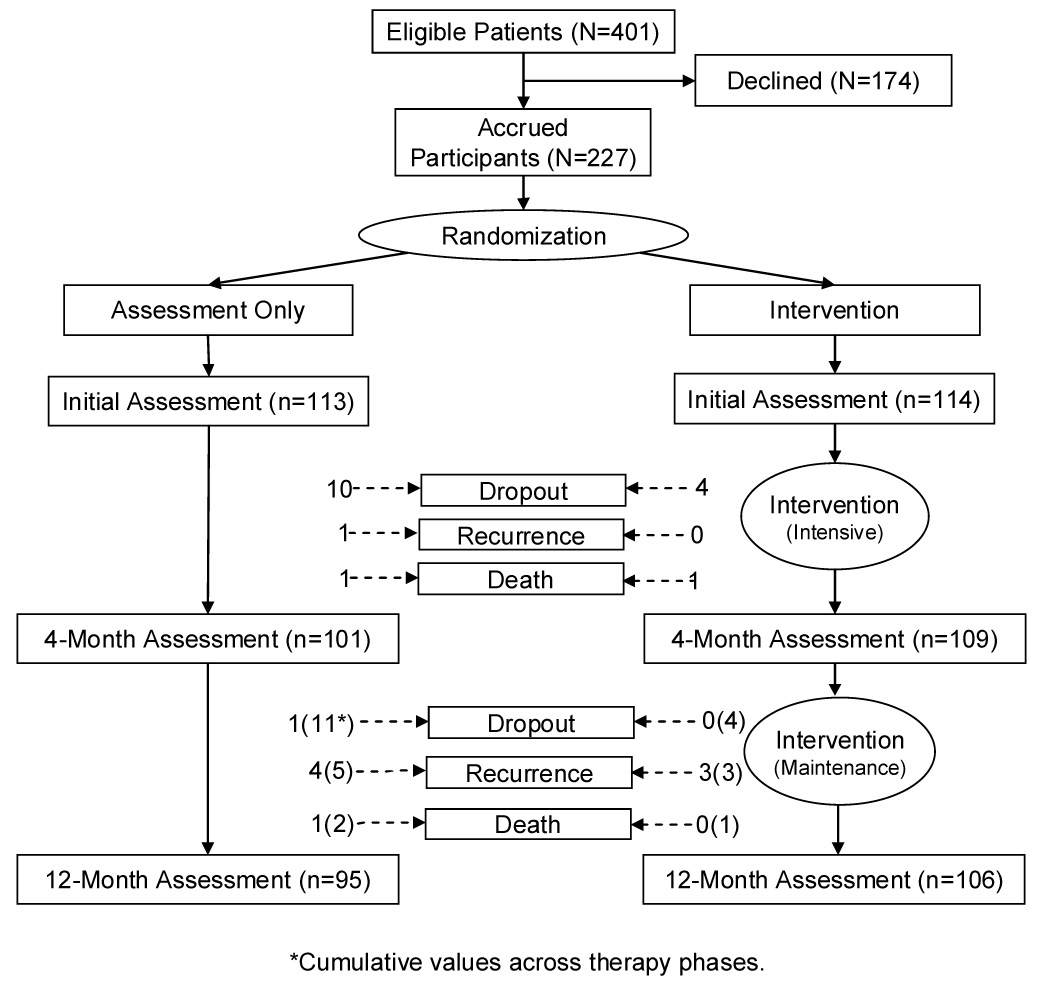
Experimental design and study flow diagram.
Table 1.
Proportions of the patients who received chemotherapy and/or radiation within four month intervals of the first year following surgery and randomization.
| Treatment | Months | Total (N=227) | Intervention (n=114) | Assessment Only (n=113) |
|---|---|---|---|---|
| Chemotherapy | 0–4 | 83% | 83% | 83% |
| 4–8 | 13% | 17% | 9% | |
| 8–12 | 0% | 0% | 0% | |
| Radiation | 0–4 | 44% | 43% | 44% |
| 4–8 | 16% | 22% | 9% | |
| 8–12 | 3% | 4% | 1% |
Study Arms
Assessment only
The initial (baseline) assessment occurred following informed consent and prior to randomization and the start of adjuvant therapy. Research assistants conducted individual interviews and administered questionnaires, and research nurses drew 60-mL of blood. At 4- and 12-months, patients were re-assessed.
Intervention and assessment
Identical interviews, questionnaires, and blood draws were completed. As described previously (Andersen et al., 2004), patients completed the intervention in small cohorts (n = 13) of approximately 8 to 12 patients. Led by two clinical psychologists, each cohort met weekly for 1.5 hours for 18 sessions during the 4-month intensive phase and then monthly for 1.5 hours for 8 sessions during the 8-month maintenance phase. The topics/techniques were similar to other psychosocial trials (Andersen, 1992; Andersen, 2002; e.g., progressive muscle relaxation, positive ways to cope, effective use of social support), but also included strategies for improving health behaviors (diet, exercise, smoking cessation) and adherence. Topics were covered systematically during the intensive phase and reviewed and monitored during maintenance.
Intervention retention was 81% (92 of 114). Of the 22 non-participants, 1 died, 3 recurred, and 4 dropped out by 12-months. The remaining 14 individuals were intervention dropouts (usually attending only one session) but continued in the trial and provided follow up data for intent to treat analyses. For the intervention participants, compliance was excellent, with patients completing 92% of the sessions (M = 24 of 26; SD = 2.32).
Measures
Cancer-related Stress and Emotional Distress
The Impact of Events Scale (IES; Horowitz et al., 1979) quantified the frequency of intrusive thoughts, denial of thoughts, and avoidant behaviors. As previously described (Andersen et al., 2004), this measure was used to determine individual differences in cancer-specific stress. Using the baseline scores, patients were categorized into Low stress (n = 114; range = 0 – 26) or High stress (n = 113; range = 27 – 65) sub groups within study arm assignment. Coefficient alpha reliability was .87 and 4-month test-retest reliability was .78.
The Profile of Mood States (POMS; McNair et al., 1971) assesses negative mood. The Total Mood Disturbance score is the sum of five scales (Anxiety, Depression, Anger, Fatigue, and Confusion) minus the score of the Vigor scale. Cronbach’s alpha reliability was .92 and 4-month test-retest reliability was .78.
Immune
Procedures for blood separation, quantification of T lymphocytes and T-cell subsets, and assay procedures have been detailed previously (Andersen et al., 2004). Briefly, isolated peripheral blood leukocytes, re-suspended in supplemented RPMI without phenol red supplemented with fetal bovine serum, were seeded in triplicate at 0.5 × 105 per well and incubated for 68 hours at 37° C. This was done in an atmosphere of 5% CO2 in 96-well flat-bottomed plates with or without PHA or Con A at 2.5, 5.0, and 10.0 µg/mL. Wells were pulsed with MTS [3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt and PMS (phenazine methosulfate)], an electron-coupling reagent, to measure proliferative responses. Proliferation was determined via optical density readings of the supernatants in the wells compared to the control, cells and media alone, using an HTS7000 Bioassay micro plate reader at a determination wavelength of 492 nm and a reference wavelength of 690 nm, as has been described (Andersen et al., 1998; Andersen et al., 2004).
Health
A research nurse, blinded to study conditions, completed two measures. Nurses conducted a detailed interview with each patient. Medical chart inspection was also used for general information, lab and diagnostic study results, etc., and the patient’s medical or surgical oncologists or oncology nurses were available for consultation.
The Karnofsky Performance Status (KPS; Karnofsky, & Burchenal, 1949) measure provided a global indicator of functional status. The scale ranges from 100 (Normal, no complaints, no evidence of disease) to 0 (Dead) with 10-point intervals, each with explicit descriptors. Lower scores indicate greater symptoms and physical restrictions. Inter-rater reliability ranges from .70 to .97 (38, 39).
Items for symptoms, signs, illnesses, lab values, exam findings, etc. came from the listing used by the Southwest Oncology Collaborative Group (SWOG; version from 1994) for clinical trials. Items are grouped within 19 body categories (hematologic/ hemorrhagic, infection, clotting disorders, circulatory, cardiac, liver, lung/pulmonary, renal/bladder, gastrointestinal, pain, neuromotor, neurosensory, dermatologic, mucosal, immunologic, flu-like, eye, metabolic, and endocrine). Each category includes 4–6 items (e.g., hypertension, hypotension, veno-occulsive disease, edema, and phlebitis/thrombosis/embolus for circulatory) rated on an item specific scale (e.g., for veno-occulsive disease the scale is 0 = none, 1 = yes; for hypotension the scale is 0 = none or no change, 1 = changes but no treatment required, 2 = requires fluid replacement or other treatment, 3 = requires treatment and hospitalization, resolves within 48 hours, and 4=requires treatment, hospitalization greater than 48 hours, or shock). Items within categories are averaged.
Analytic Strategy
Descriptive analyses test for significant health and biobehavioral changes at the 12-month follow-up, using the same repeated measures analysis of variance (ANOVA) statistics as the original report (Andersen et al., 2004). ANOVAs first test for the combination of the intervention’s intensive and maintenance phases on 12-month health, with Study Arm (Intervention vs. Assessment only) as the between subjects factor and Time (Initial, 12-month) as the within subjects factor. For the Emotional distress (POMS) outcome, Initial Cancer Stress (Low vs. High) is included as an additional between subjects factor, as previously described (Andersen et al., 2004). As the immune outcomes exhibit low within-group pre treatment-post treatment correlations, the multivariate analysis of covariance (MANCOVA) model is best suited to reduce the error term and provide greater power (Girden, 1992). Any significant MANCOVA is followed by univariate ANCOVAs for the three PHA and ConA concentration levels (2.5, 5.0, and 10.0) for each assay.
Linear path analysis models the relationships between study arm, emotional distress, immunity, and health across time. Variables in the model serve as predictors, outcomes, or mediator(s) of indirect relationships between predictors and outcomes. The specified path model (see Figure 3) tests the direct effect of the intervention on health status at 12 months, as well as the indirect effects observed at 4 months through (a) distress reduction and/or (b) increases in immune function. Specifically, a direct intervention effect is estimated using the path between Study Arm and 12-month Health Status. In addition, two indirect paths are estimated: one from 4-month Emotional Distress to Health Status at 12-months, and the other from 4-month Immunity to Health Status at 12-months. Finally, all the remaining paths are control paths, each accounting for any effect of initial (baseline) values for Health Status, Emotional Distress, Cancer Stress, or Immunity on 12-month Health Status. Results provide standardized estimates of path weights, indicating the magnitude, direction (positive or inverse), and significance of relationships between variables. The overall “fit” of the model is evaluated in terms of its ability to account for the pattern of correlations observed among the variables, using the Root Mean Square Error of Approximation (RMSEA) statistic.
Fig. 3.
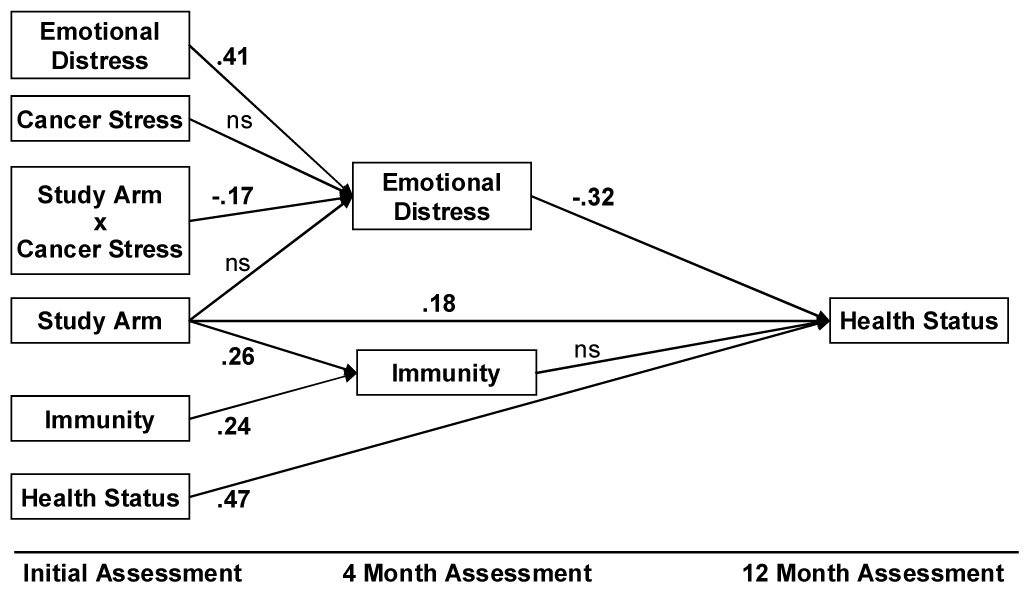
Path model of longitudinal relationships among Cancer Stress, psychological intervention (Study Arm), Cancer Stress × Study Arm interaction, Emotional Distress, Immunity, and Health Status outcomes. The model accounts for initial levels of Emotional Distress, Immunity, and Health Status. Resulting path weights are provided where statistically significant (p < .05). Study Arm had a positive effect on 4-month Immunity. Study Arm also interacted with initial cancer-related stress (Study Arm × Cancer Stress), producing greater reductions in 4-month Emotional Distress for intervention women with high initial levels of Cancer Stress. By 12 months, Study Arm had a direct positive effect on Health Status. Four-month Emotional Distress, but not 4-month Immunity, was a significant predictor of Health Status. The indirect effect of the Study Arm × Cancer Stress interaction on 12-month Health Status via 4-month Emotional Distress was significant (z = 2.11, p = .04).
To increase the generality of the test, composite indices for immune and health measures are calculated from multiple measures. The Immunity composite combines the three T-cell proliferation levels for both ConA and PHA so that higher scores indicate stronger proliferative responses. The Health composite similarly combines the KPS and the symptoms/signs measures with equal weight; higher scores indicate better functional status and fewer symptoms/signs. Analyses were conducted to empirically determine the psychometric homogeneity of the two health measures. Exploratory factor analysis was used and included the KPS score and the 19 category scores of the symptoms/signs measure. Maximum Likelihood discrepancy function and oblique Crawford-Ferguson varimax rotation were used with the Comprehensive Exploratory Factor Analysis program (CEFA; Browne et al., 1998). After an inspection of eigenvalues and scree plot, one and two factor solutions were extracted. Item loadings, factor interpretability, and goodness of model fit were next evaluated. The two-factor solution showed poor specificity of factor structure in that some items had high loadings on both factors and several other items showed low loadings on both factors. In contrast, the single factor solution was interpretable and had a satisfactory fit (RMSEA = .065, 90% CI = .053 – .077). Thus, psychometric structure for the health status outcome is unidimensional and includes the KPS and the 19 categories noted.
RESULTS
Data Availability
Excluding the 11 cases of recurrence/death (11 of 227; 5%), there was 93% retention (201 of 216) at 12-months (see Figure 1). Retention and availability of data was equivalent between study arms. Specifically, complete data were available from the initial assessment. At 12-months, data were available from 91% of the patients for Stress (IES) and Emotional distress (POMS) measures, from 92% for the Karnofsky Performance Status (KPS), from 91% for the symptoms/signs, and from 79% for the ConA and PHA assays. The lower percentage for the latter was due to low blood draw amounts and/or cell counts. The path analysis was conducted with 145 patients who had complete data for all the variables in the model. We note that analyses comparing the participants who had complete data (n = 145) to the remainder (n = 82) were conducted using chi-square or ANOVA as appropriate. The groups did not significantly differ in sociodemographics, disease characteristics, or cancer treatment received (ps > .07).
Descriptive Analyses Testing for Improved Health and Biobehavioral Effects
As predicted, the intervention resulted in better health outcomes; significant Group × Time interaction effects were found for both health measures (see Figure 2). On the KPS, the Assessment group was evaluated as more functionally impaired and restricted in their daily activities, including self-care [ANOVA, F(1,183) = 7.19, p = .008, ηp² = .04]. Over the 12 month observation interval, functional status increased by 7% (83 to 89) in the Intervention arm but by only 1% (87 to 88) in the Assessment arm. On the symptoms/signs, there was a significant interaction [ANOVA, F(1,175) = 3.81, p = .05, ηp² = .02]. Over the 12-month observation interval, toxicities/symptoms increased by 29% (0.21 to 0.27) in the Assessment arm but only 14% (0.22 to 0.25) in the Intervention arm.
Fig. 2.
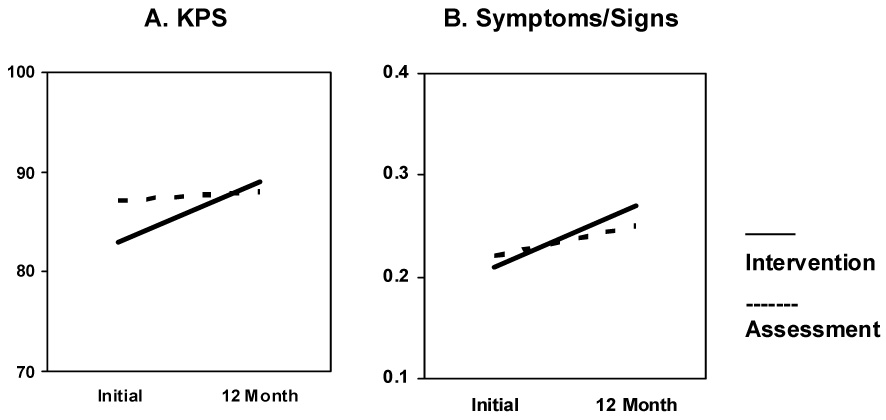
Significant Group × Time interaction effects for the Karnofsky Performance Status (KPS) and Symptoms/Signs.
We also note significant biobehavioral effects. For the POMS, a significant three-way (Cancer Stress × Study Arm × Time) interaction was found and indicated that the effect of the intervention on Total Mood Disturbance at 12-months depended upon initial levels of cancer stress [ANOVA, F(1,179) = 4.02, p < .05, ηp² = .02]. That is, for patients who began the trial with high cancer-specific stress, declines in Total Mood Disturbance were significantly greater among Intervention patients than among Assessment patients at 12-months. Also as predicted, PHA blastogenesis was significantly higher for the Intervention group [MANCOVA, F(3,145) = 3.86, p = .01, ηp² = .07]. The follow-up ANCOVAs for the three PHA dilutions were significant (all ps ≤ .04, ηp²s ≥ .03) with a similar pattern for each; proliferation remained relatively constant for the Intervention arm but declined for the Assessment only arm. Intervention effects on ConA blastogenesis, apparent after the intensive phase (Andersen et al., 2004), were not significant after the 12-month maintenance phase. [Note: Prior to conducting all immune analyses, we determined that groups did not differ on T-cell counts (T3, T4, and T8, ps > .44)].
Path Analyses Testing for Direct and Indirect Intervention Effects on Health
The Figure 3 path model was evaluated using LISREL 8.71 software with N=145 patients with complete data. The hypothesized model adequately fit the observed data [χ²(11) = 16.39, p = .13; RMSEA = .06; CFI = .97; GFI = .98)]. Moreover, the model explained significant, substantial variance--36%--in 12-month Health Status. [Note: With initial Health Status removed from the model, 20% of the variance in 12-month Health Status was still explained.] In Figure 3, all statistically significant path weights are provided in standardized form. The left portion of the model repeats the findings from the earlier report (Andersen et al., 2004) and shows the direct, positive effect of the intervention (Study Arm) on 4-month Immunity, and the interaction between study arm and cancer-related stress (Study Arm × Cancer Stress) on reducing Emotional Distress at 4-months.
More important for the present analysis is the prediction of Health Status at 12-months. First, Study Arm had a direct positive effect as hypothesized, on 12-month Health Status, indicated by the significant .18 path weight (z = 2.51, p = .01). Secondly, we tested the two pathways (mechanisms) for achieving positive health. Support was found for a significant, indirect effect of distress reduction on health (z = 2.11, p = .04). That is, for those women in the intervention arm with the highest distress (Study arm × Cancer Stress), improved Health Status at 12-months occurred via the mechanism of Emotional Distress reduction which occurred at 4-months. This reduction was important, because Emotional Distress had a negative effect on 12-month Health Status, as indicated by the −.32 path weight (z = −4.67, p < .001).
In contrast, there was no significant indirect effect via Immunity. Although there was a positive effect of Study Arm on 4-month Immunity, as indicated by the .26 path weight (z = 3.21, p = .002) between the variables, the changes in Immunity at 4 months did not predict Health Status at 12 months; the path weight between the two variables was not significant.
Finally, all significant paths remained when statistically controlling for baseline levels of Distress, Immunity, and Health, as indicated. Other control variables (i.e., age/menopausal status, partner status, chemotherapy, and hormonal therapy) were tested and the findings were identical (data not shown).
DISCUSSION
Results show that an effective psychological intervention—one achieving significant changes in psychological and immune measures—can also improve health. In this study health was defined both with global ratings of the patient’s performance status and specific ratings of common signs and symptoms of medical conditions and illnesses, laboratory studies, and possible toxicities from chemotherapy and radiation therapy regimens. Path analyses were used and the model explained 36% of the variance in health at 12 months. The intervention had both a direct effect and an indirect effect; by specifically lowering the patients’ levels of emotional distress, the health outcomes were more positive. Although the intervention was also associated with positive immune responses, these changes were unrelated to subsequent changes in health status.
The higher functional status and lower level of symptomatology, toxicities, etc. for the intervention group is clinically important. Characteristics of the present trial—disease endpoints as primary outcomes and tests of mechanisms for an effect—made it important to have longitudinal documentation of the patients’ general health. Search measures in several literatures were unproductive or unsatisfactory. Thus, we turned to the cancer treatment clinical trial studies for solutions. Because of the predominance of ongoing SWOG clinical trials at our institution at the time, their listing was chosen, as many of our patients would be participating in trials for which this listing was designed. Indeed, after disease markers, these data become the secondary endpoints in Phase III trials. When treatments are compared and found to be equally efficacious, these data become, for many patients and physicians alike, the basis for choosing among treatments (see Potosky et al., 1999 for data for prostate cancer; see discussion in Goodwin, et al., 2003, for breast cancer). In addition, these health data provide guidance for dosage modification in Phase II trials (Pelegri et al., 2005).
The health measures in this study also have importance as they covary with behavioral and psychological variables. The magnitude of treatment toxicities has been used to determine risk for poor adherence to treatment (Ayres et al., 1994; Levin et al., 1999; Demissie et al., 2001). Knowing the extent of a slowed recovery and/or disease and treatment morbidities is important for caring for survivors. Poor health status has a negative effect on mood (Ganz et al., 2004) and quality of life (Ganz et al., 2004; Levin et al., 1999), and health worries are the source of greatest concern among survivors (Spencer et al., 1999), as poor health negatively impacts personal relationships (Wimberly et al., 2005), daily activity levels, and return to normal routines such as employment (Bradley et al., 2005). In addition, poorer health impacts the meaning patients derive from life following cancer (Jim & Andersen, in press).
While some psychological trials have used the KPS (Dodd, 1988; Northouse et al., 2005; Scheier et al., 2005) the use of an extensive symptomatology and toxicity listing is novel. These measures are costly, as a high level of rater expertise is required (e.g., a nurse specialist), and they are time consuming with the patient interview, evaluating lab results and chart data, etc. Yet, they provide significant breadth, relevance, and with symptom-specific graded scales, objectivity. These measures are different in content and method from self-reported perceptions of health or symptoms. Correlations between patients’ reports of emotional distress and evaluations of their physical health may be inflated from shared method variance (i.e., both self-report measures). In addition to the objectivity of the items used here, nurses blinded to the patients’ study arm assignment were the raters. These factors greatly reduce measurement error related to the patient (e.g., reporting bias).
A second aim of this report was to identify how this psychological intervention could achieve positive health effects. While there are many possible routes, two likely mechanisms were tested. The path model accounted for the contribution of distress reduction, per se, on health status. In addition, the direct effect of the intervention suggested that additional mechanisms also played a positive role. The intervention included multiple components, and we can only speculate about which factors may be influential. Progressive muscle relaxation (PMR) provided patients with an active, and portable, means to reduce daily stress and cope with and control symptoms (e.g., nausea, disturbed sleep, fatigue). In other studies with cancer patients, PMR has yielded reductions in anxiety (Lyles et al., 1982) and elevations in immune measures (Lekander et al., 1997). Other intervention components also may have had general health benefits also; possibilities include recommendations for regular exercise [which has had symptom reducing effects in other studies with breast cancer patients (e.g., MacVicar et al., 1989)] and teaching patients to use direct, assertive communications with their physicians and nurses to get their medical needs met. Future studies will explore these and other possibilities.
There is great research interest in the potential for interrelationships among stress, immunity, and health (Kiecolt-Glaser et al., 2002). It is suggested that a robust immune response might maintain health by lowering one’s risk for infection or illness (Janeway, 2004), for example. While there is correlational evidence for the covariation of heightened stress, lowered immunity, and selected health outcomes (Kiecolt-Glaser et al., 2002), there are few experimental data in which stress is ‘manipulated’ (via randomization to an intervention) and the consequent effect on immunity and health are tested. To our knowledge, only Fawzy et al. (1993) provided relevant data, though not a test of relationships among the three variables. They reported that lower levels of baseline distress predicted recurrence and death, and higher natural killer cell activity predicted a lower risk for recurrence, though not survival.
In the present study, the intervention-produced changes in blastogenesis levels were statistically significant, but did not relate to health measures. [We also note that the effect size for the PHA observed at 12 months was ‘medium’ to ‘large’ by accepted standards (Cohen, 1988) and compares favorably with other estimates. In a meta analysis of psychological interventions and immunity, effect sizes for blastogenesis measures were small (rs of −.05 for ConA and .13 for PHA (Miller and Cohen, 2001)]. However, our health outcome assessed many bodily systems, a few of which likely had more direct relevance to immunity (‘infection’) than several of the others. Studying a health outcome particularly relevant for T-cell function and selection of assays with a direct link to a specific health outcome (Lutgendorf et al., 2002), may provide additional information regarding this hypothesized mechanism.
In summary, results show that a psychological intervention yielding robust biobehavioral effects also improved health. This was achieved, in part, through lowering of emotional distress rather than the seemingly positive effect that the intervention had on T-cell immunity. Ten-year follow-up are being collected to determine whether health status of these patients is related to disease progression, as the survivorship period brings new challenges for cancer patients (Yabroff et al., 2004).
Supplementary Material
Acknowledgments
Research support: American Cancer Society (PBR-89), the Longaberger Company-American Cancer Society Grant for Breast Cancer Research (PBR-89A; RSGPB-03-248-01-PBP), the U.S. Army Medical Research Acquisition Activity Grants (DAMD17-94-J-4165, DAMD17-96-1-6294, and DAMD17-97-1-7062), National Institute of Mental Health (RO1MH51487), and National Cancer Institute (RO1CA92704, KO5 CA098133, P30 CA16058).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- Allen SM, Shah AC, Nezu AM, Nezu CM, Ciambrone D, Hogan J, Mor V. A problem-solving approach to stress reduction among younger women with breast carcinoma. Cancer. 2002;94:3089–3100. doi: 10.1002/cncr.10586. [DOI] [PubMed] [Google Scholar]
- Andersen BL. Psychological interventions for cancer patients to enhance the quality of life. J. Consult. Clin. Psychol. 1992;60:552–568. doi: 10.1037//0022-006x.60.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL. Biobehavioral outcomes following psychological interventions for cancer patients. J. Consult. Clin. Psychol. 2002;70:590–610. doi: 10.1037//0022-006X.70.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz D, Kutz LA, MacCallum R, Courtney ME, Glaser R. Stress and immune responses after surgical treatment for regional breast cancer. J. Natl. Cancer Inst. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, Shapiro CL, Carson WE., 3rd Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J. Clin. Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Amer. Psychol. 1994;49:389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres A, Hoon PW, Cotanch PH, Franzoni JB, Takayanagi S, Matheny KB. Influence of mood and adjustment to cancer on compliance with chemotherapy among breast cancer patients. J. Psychosom. Res. 1994;38:393–402. doi: 10.1016/0022-3999(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Bradley CJ, Neumark D, Luo Z, Bednarek H, Schenk M. Employment outcomes of men treated for prostate cancer. J. Natl. Cancer Inst. 2005;97:958–965. doi: 10.1093/jnci/dji171. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R, Tateneni K, Mels G. CEFA: Comprehensive Exploratory Factor Analysis. [Software] Columbus, OH: The Ohio State University; 1998. [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J. Clin. Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1988. [Google Scholar]
- Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J. Clin. Oncol. 2001;19:322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- de Wit R, van Dam F, Zandbelt L, van Buuren A, van der Heijden K, Leenhouts G, Loonstra S. A pain education program for chronic cancer pain patients: follow-up results from a randomized controlled trial. Pain. 1997;73:55–69. doi: 10.1016/s0304-3959(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Dodd MJ. Efficacy of proactive information on self-care in chemotherapy patients. Patient Educ. Couns. 1988;11:215–225. doi: 10.1016/0738-3991(88)90021-3. [DOI] [PubMed] [Google Scholar]
- Doorenbos A, Given B, Given C, Verbitsky N. Physical functioning: effect of behavioral intervention for symptoms among individuals with cancer. Nurs. Res. 2006;55:161–171. doi: 10.1097/00006199-200605000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar L, Rosberger Z, Collet JP. Lessons learned: outcomes and methodology of a coping skills intervention trial comparing individual and group formats for patients with cancer. Int. J. Psychiatry Med. 2001;31:289–304. doi: 10.2190/U0P3-5VPV-YXKF-GRG1. [DOI] [PubMed] [Google Scholar]
- Edmonds CVI, Lockwood GA, Cunningham AJ. Psychological response to long term group therapy: a randomized trial with metastatic breast cancer patients. Psychooncology. 1999;8:74–91. doi: 10.1002/(SICI)1099-1611(199901/02)8:1<74::AID-PON339>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Fawzy FI, Fawzy NW, Hyun CS, Elashoff R, et al. Malignant melanoma: effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch. Gen. Psychiatry. 1993;50:681–689. doi: 10.1001/archpsyc.1993.01820210015002. [DOI] [PubMed] [Google Scholar]
- Forester B, Kornfeld DS, Fleiss JL. Psychotherapy during radiotherapy: effects on emotional and physical distress. Am. J. Psychiatry. 1985;142:22–27. doi: 10.1176/ajp.142.1.22. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, Bower JE, Belin TR. Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J. Natl. Cancer Inst. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- Giesler RB, Given B, Given CW, Rawl S, Monahan P, Burns D, Azzouz F, Reuille KM, Weinrich S, Koch M, Champion V. Improving the quality of life of patients with prostate carcinoma. Cancer. 2005;104:752–762. doi: 10.1002/cncr.21231. [DOI] [PubMed] [Google Scholar]
- Girden ER. ANOVA: Repeated measures. Newbury Park, CA: Sage; 1992. [Google Scholar]
- Golden-Kreutz DM, Thornton LM, Wells-Di Gregorio S, Frierson GM, Jim HS, Carpenter KM, Shelby RA, Andersen BL. Traumatic stress, perceived global stress, and life events: Prospectively predicting quality of life in breast cancer patients. Health Psychol. 2005;24:288–296. doi: 10.1037/0278-6133.24.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin PJ, Black JT, Bordeleau LJ, Ganz PA. Health-related quality-of-life measurement in randomized clinical trials in breast cancer--taking stock. J. Natl. Cancer Inst. 2003;95:263–281. doi: 10.1093/jnci/95.4.263. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Leszcz M, Ennis M, Koopmans J, Vincent L, Guther H, Drysdale E, Helgeson VS, Cohen S, Schulz R, Yasko J. Education and peer discussion group interventions and adjustment to breast cancer. Arch. Gen. Psychiatry. 1999;56:340–347. doi: 10.1001/archpsyc.56.4.340. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom. Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Janeway C. Immunobiology. New York: Garland Science; 2004. [Google Scholar]
- Johnson JE, Nail LM, Lauver D, King KB, Keys H. Reducing the negative impact of radiation therapy on functional status. Cancer. 1988;61:46–51. doi: 10.1002/1097-0142(19880101)61:1<46::aid-cncr2820610109>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Jim H, Andersen B. Meaning in life mediates the relationship between social and physical functioning and distress in cancer survivors. Br. J. Health Psychol. doi: 10.1348/135910706X128278. in press. [DOI] [PubMed] [Google Scholar]
- Karnofsky D, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod C, editor. Evaluation of chemotherapeutic agents; symposium held at the New York Academy of Medicine; March 25 and 26, 1948; New York: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: Psychological influences on immune function and health. J. Consult. Clin. Psychol. 2002;70:537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- Lekander M, Furst CJ, Rostein S, Hursti TJ, Fredrikson M. Immune effects of relaxation during chemotherapy for ovarian cancer. Psychother. Psychosom. 1997;66:185–191. doi: 10.1159/000289133. [DOI] [PubMed] [Google Scholar]
- Levin M, Mermelstein H, Rigberg C. Factors associated with acceptance or rejection of recommendation for chemotherapy in a community cancer center. Cancer Nurs. 1999;22:246–250. doi: 10.1097/00002820-199906000-00009. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Johnson EL, Cooper B, Anderson B, Sorosky JI, Buller RE, Sood AK. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- Lyles JN, Burish TG, Krozely MG, Oldham RK. Efficacy of relaxation training and guided imagery in reducing the aversiveness of cancer chemotherapy. J. Consult. Clin. Psych. 1982;50:509–524. doi: 10.1037//0022-006x.50.4.509. [DOI] [PubMed] [Google Scholar]
- MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients' functional capacity. Nurs. Res. 1989;38:348–351. [PubMed] [Google Scholar]
- Maguire P, Brooke M, Tait A, Thomas C, Sellwood R. The effect of counseling on physical disability and social recovery after mastectomy. Clin. Oncol. 1983;9:319–324. [PubMed] [Google Scholar]
- Maguire P, Parkes CM. Surgery and loss of body parts. B.M.J. 1998;316:1086–1088. doi: 10.1136/bmj.316.7137.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, Glare P, Nabal M, Vigano A, Larkin P, De Conno F, Hanks G, Kaasa S. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations--a study by the Steering Committee of the European Association for Palliative Care. J. Clin. Oncol. 2005;23:6240–6248. doi: 10.1200/JCO.2005.06.866. [DOI] [PubMed] [Google Scholar]
- McCorkle R, Benoliel JQ, Donaldson G, Georgiadou F, Moinpour C, Goodell B. A randomized clinical trial of home nursing care for lung cancer patients. Cancer. 1989;64:1375–1382. doi: 10.1002/1097-0142(19890915)64:6<1375::aid-cncr2820640634>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. EITS Manual of the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- Miller GE, Cohen S. Psychological interventions and the immune system: a metaanalytic review and critique. Health Psychol. 2001;20:47–63. doi: 10.1037//0278-6133.20.1.47. [DOI] [PubMed] [Google Scholar]
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Jaenicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Chaudri-Ross H, Lang R, Wyld P, Bhatnagar A. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J. Clin. Oncol. 2003;21:2101–2109. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- Mouridsen H, Sun Y, Gershanovich M, Perez-Carrion R, Becquart D, Chaudri-Ross HA, Lang R. Superiority of letrozole to tamoxifen in the first-line treatment of advanced breast cancer: evidence from metastatic subgroups and a test of functional ability. Oncologist. 2004;9:489–496. doi: 10.1634/theoncologist.9-5-489. [DOI] [PubMed] [Google Scholar]
- Northouse L, Kershaw T, Mood D, Schafenacker A. Effects of a family intervention on the quality of life of women with recurrent breast cancer and their family caregivers. Psychooncology. 2005;14:478–491. doi: 10.1002/pon.871. [DOI] [PubMed] [Google Scholar]
- Potosky AL, Harlan LC, Stanford JL, Gilliland FD, Hamilton AS, Albertsen PC, Eley JW, Liff JM, Deapen D, Stephenson RA, Legler J, Ferrans CE, Talcott JA, Litwin MS. Prostate cancer practice patterns and quality of life: the Prostate Cancer Outcomes Study. J. Natl. Cancer Inst. 1999;91:1719–1724. doi: 10.1093/jnci/91.20.1719. [DOI] [PubMed] [Google Scholar]
- Pelegri A, Calvo L, Anton A, Mayordomo JI, Florian J, Vasquez S, Arcusa A, Martin-Richard M, Bayo JL, Carrasco E, Virizuela J. Docetaxel/gemcitabine administered every other week as first-line treatment for metastatic breast cancer: Final results of a phase II trial. Clin. Breast Cancer. 2005;6:433–438. doi: 10.3816/cbc.2005.n.048. [DOI] [PubMed] [Google Scholar]
- Quesnel C, Savard J, Simard S, Ivers H, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J. Consult. Clin. Psychol. 2003;71:189–200. [PubMed] [Google Scholar]
- Roscoe JA, Morrow GR, Hickok JT, Stern RM. Nausea and vomiting remain a significant clinical problem: trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in community clinical practices. J. Pain Symptom Manage. 2000;20:113–121. doi: 10.1016/s0885-3924(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Helgeson VS, Schulz R, Colvin S, Berga S, Bridges MW, Knapp J, Gerszten K, Pappert WS. Interventions to enhance physical and psychological functioning among younger women who are ending nonhormonal adjuvant treatment for early-stage breast cancer. J. Clin. Oncol. 2005;23:4298–4311. doi: 10.1200/JCO.2005.05.362. [DOI] [PubMed] [Google Scholar]
- Shapiro CL, Recht A. Drug therapy: Side effects of adjuvant treatment of breast cancer. N. Engl. J. Med. 2001;344:1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- Spector PE, Jex SM. Development of four self-report measures of job stressors and strain: Interpersonal Conflict at Work Scale, Organizational Constraints Scale, Quantitative Workload Inventory, and Physical Symptoms Inventory. J. Occup. Health. Psych. 1998;3:356–367. doi: 10.1037//1076-8998.3.4.356. [DOI] [PubMed] [Google Scholar]
- Spencer SM, Lehman JM, Wynings C, Arena P, Carver CS, Antoni MH, Derhagopian RP, Ironson G, Love N. Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychol. 1999;18:159–168. doi: 10.1037//0278-6133.18.2.159. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Bloom JR. Group therapy and hypnosis reduce metastatic breast cancer pain. Psychosomatic Medicine. 1983;45:333–339. doi: 10.1097/00006842-198308000-00007. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2:888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Danoff-Burg S, Sworowski LA, Collins CA, Branstetter AD, Rodriguez-Hanley A, Kirk SB, Austenfeld JL. Randomized, controlled trial of written emotional expression and benefit finding in breast cancer patients. J. Clin. Oncol. 2002;20:4160–4168. doi: 10.1200/JCO.2002.08.521. [DOI] [PubMed] [Google Scholar]
- Ware J, Snow K, Kosinski M. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: Quality Metric Incorporated; 2000. [Google Scholar]
- Wimberly SR, Carver CS, Laurenceau J, Harris SD, Antoni MH. Perceived partner reactions to diagnosis and treatment of breast cancer: impact on psychosocial and psychosexual adjustment. J. Consult. Clin. Psych. 2005;73:300–311. doi: 10.1037/0022-006X.73.2.300. [DOI] [PubMed] [Google Scholar]
- Yabroff KR, Lawrence WF, Claurser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population-based national sample. J. Natl. Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- Yates P, Aranda S, Hargraves M, Mirolo B, Clavarino A, McLachlan S, Skerman H. Randomized controlled trial of an educational intervention for managing fatigue in women receiving adjuvant chemotherapy for early-stage breast cancer. J. Clin. Oncol. 2005;23:6027–6036. doi: 10.1200/JCO.2005.01.271. [DOI] [PubMed] [Google Scholar]
- Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky Performance Status. Cancer. 1980;45:2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Yates JW, McKegney FP, Kun LE. Proceedings of the National Conference on Human Values and Cancer. New York: American Cancer Society; 1981. A comparative study of home nursing care of patients with advanced cancer; pp. 207–218. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Excluding the 11 cases of recurrence/death (11 of 227; 5%), there was 93% retention (201 of 216) at 12-months (see Figure 1). Retention and availability of data was equivalent between study arms. Specifically, complete data were available from the initial assessment. At 12-months, data were available from 91% of the patients for Stress (IES) and Emotional distress (POMS) measures, from 92% for the Karnofsky Performance Status (KPS), from 91% for the symptoms/signs, and from 79% for the ConA and PHA assays. The lower percentage for the latter was due to low blood draw amounts and/or cell counts. The path analysis was conducted with 145 patients who had complete data for all the variables in the model. We note that analyses comparing the participants who had complete data (n = 145) to the remainder (n = 82) were conducted using chi-square or ANOVA as appropriate. The groups did not significantly differ in sociodemographics, disease characteristics, or cancer treatment received (ps > .07).


