Figure 3.
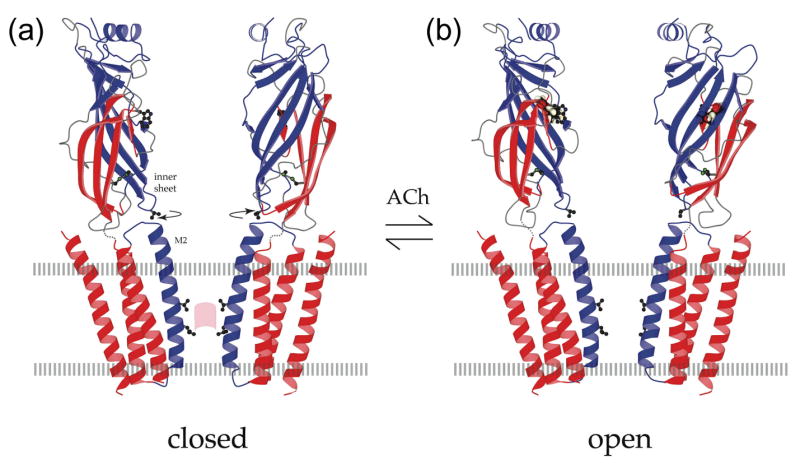
Open and closed states of the acetylcholine receptor. (a) Atomic model of the acetylcholine receptor in the closed conformation based on a 4Å density map [9]. (b) Binding of acetylcholine opens the channel by initiating rotational movements (arrows) of the inner β-sheets of the α subunits in the ligand-binding domain. These movements are communicated to the inner (M2) helices lining the pore and break apart the gate – a hydrophobic girdle in the middle of the membrane – so that ions can flow through. A tryptophan side chain in the ligand-binding domain identifies the acetylcholine-binding region; a valine side chain links the inner sheet to the inner helix; leucine and valine side chains on the inner helices make the gate (pink patch); the locations of the membrane surfaces are indicated by broken lines; the relevant moving parts are in blue. Figure reprinted by permission of Federation of the European Biochemical Societies from “Structure and action of the nicotinic acetylcholine receptor explored by electron microscopy” by Nigel Unwin, FEBS Letters, 555, 91–95, 2003.
