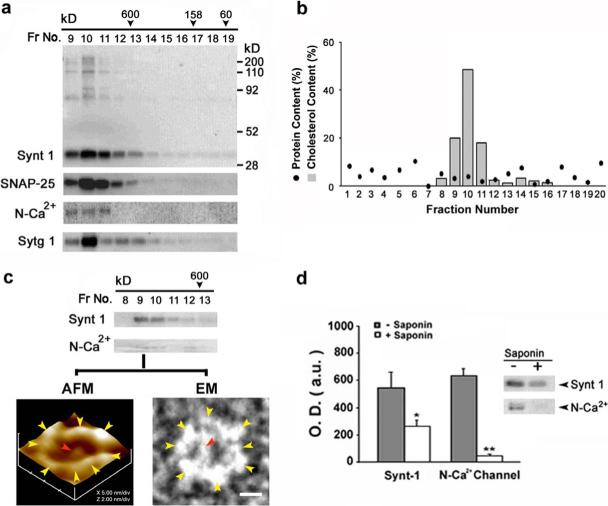
Figure 3. Cholesterol at the synaptosomal membrane is required to maintain the integrity of the neuronal fusion pore.
The elution profile of various fusion pore proteins (a), and cholesterol (b) in solubilized synaptosomal membrane preparation resolved on a G-200 sizing column, is demonstrated. Synaptosomal membrane were solubilized in Triton / Lubrol (1% w/v) PBS, and loaded onto Sephadex G-200 gel filtration column. Fractions 1 through 20 were collected and assayed for various fusion pore proteins following SDS-PAGE and immunoblot analysis. Note the elution Syntaxin 1 (Synt 1), SNAP-25, N-type calcium channel (N-Ca2+), and synaptotagmin (Sytg 1), primarily in fractions 9−11, a >650 kDa complex. Similarly, cholesterol elutes primarily in the same fractions (9−11), as assayed using Sigma Infinity cholesterol reagent kit. (c) When SNAP-25 -immunoisolated fusion pores from Triton-Lubrol-solubilized synaptosomal membrane was resolved using the column, the fusion pore complex eluted primarily in fractions 9 and 10, as demonstrated from immunoanalysis of the fractions for Syntaxin 1 and N-type Ca2+-channel. This is further confirmed by both atomic force microscopy (AFM) of the lipid-reconstituted fractions, and negative-staining electron microscopy (EM) of the fractions. Only fractions 9 and 10 contained the intact fusion pore as demonstrated (c). Note the 10−12 nm protein backbone of the fusion pore in the EM, demonstrating an asymmetric cart-wheel structure. (d) SNAP-25-immunoisolation of cholesterol-depleted (using saponin) synaptosomal membrane preparations, demonstrate a significant abrogation (*P<0.05; **P<0.01) of SNAP-25 binding to Syntaxin 1 and the N-type Ca2+-channel.