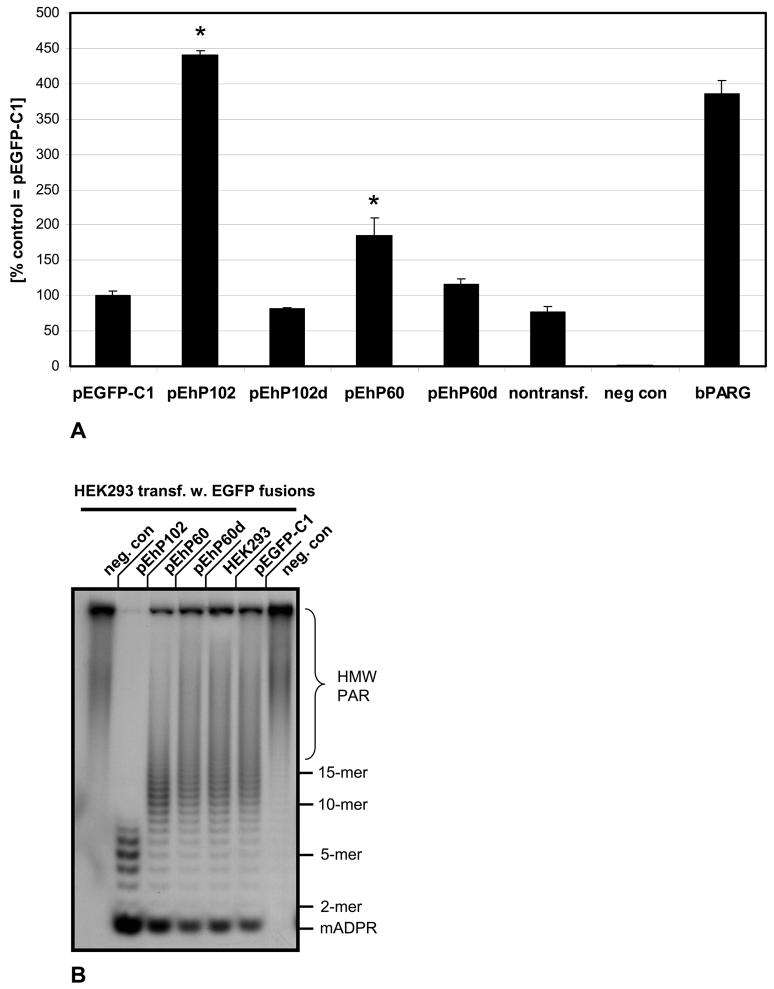
Fig. 6. Human hPARG60 is enzymatically active.
In vitro poly(ADP-ribose) glycohydrolase assays using 32P labeled PAR were employed to measure relative exoglycosidic PARG activities in extracts of transiently transfected HEK293 cells expressing EGFP-hPARG fusion proteins (A). PARG activity in extracts of HEK293 cells transfected with the parental vector pEGFP-C1 was defined as 100% and the scale reflects the percentage of PARG activity in the other samples relative to this control. Nontransfected cells and cells transfected with pEhP102d, encoding an enzymatically dead mutant (E756N in full-length hPARG111) of PARG102 generated by site-directed mutagenesis, were used as controls. The negative control samples (“neg. con.”) contained no protein (background). Positive controls used 6.75 ng of recombinant bovine PARG. Significance analyses using pooled data from three independent experiments were done using the Student's T-test (p<0.005).
(B) Aliquots of samples measured in PARG assays (A, see above) were subjected to ADPR polymer PAGE autoradiograph analyses to visualize 32P labeled products of poly(ADP-ribose) digests. Data were pooled from three independent experiments each carried out at least in quadruplicates for these analyses and a representative autoradiograph is shown.