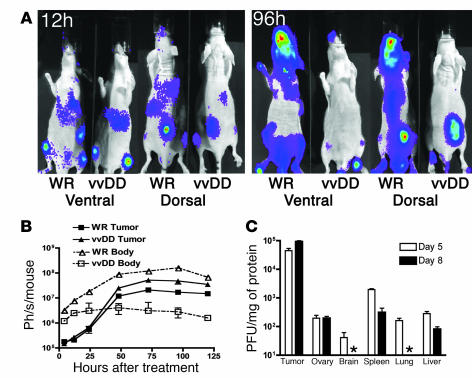
Figure 6. Intravenous delivery and tumor selectivity of vvDD versus wild-type vaccinia (WR).
(A) Biodistribution of WR and vvDD following systemic delivery to tumor-bearing mice. Athymic CD1 nu/nu mice bearing subcutaneous human HCT 116 tumors (arrows) were treated with 1 × 107 PFU of vaccinia strains via tail vein injection. Viral strains (WR and vvDD) expressed luciferase, and the subsequent biodistribution of viral gene expression was detected by bioluminescence imaging in an IVIS 100 system (Xenogen; Caliper Life Sciences) following addition of the substrate luciferin at the times indicated after treatment. Representative mice from n = 5/group are shown; the remaining mice are shown in Supplemental Figure 6. (B) Viral gene expression, as quantified by light production, was plotted over time for the regions of interest covering the whole body (ventral image) (dashed line, open symbols) or from the tumor only (dorsal view, region of interest over tumor) (solid line, filled symbols) for BALB/c mice bearing subcutaneous JC tumors (n = 5 mice/group) and treated with 1 × 107 PFU of either virus by tail vein injection. (C) Recovery of vvDD delivered systemically (i.p. injection of 1 × 109 PFU) to C57BL/6 mice bearing subcutaneous MC38 tumors. Mice were sacrificed on days 5 or 8 after treatment (n = 8/group), different tissues recovered, and viral infectious units (PFU/mg tissue) titered by plaque assay (asterisk indicates below the limits of detection).