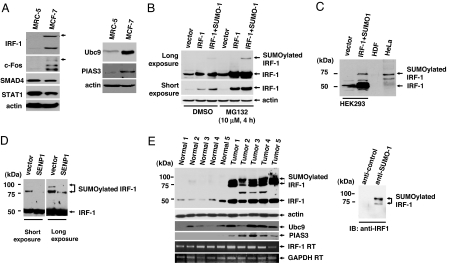
Fig. 1.
SUMOylation of IRF-1 in cell lines and tumors. (A) MRC-5 and MCF-7 cells were lysed with SDS-loading buffer, and equal amounts of cell lysates were immunoblotted with the indicated antibodies. (B) IRF-1 is SUMOylated after coexpression with SUMO-1. HEK293 cells were transiently transfected with plasmids encoding IRF-1 and FLAG-SUMO-1. Treatment with the proteasomal inhibitor, MG132, blocked IRF-1 degradation. (C) The size of the shifted band in HeLa cells was similar to that of SUMOylated IRF-1. The SUMOylated IRF-1 band in HEK293 was used as a positive control. (D) SENP1 cleaved the bond between IRF-1 and SUMO-1. HeLa cells were transiently transfected with a control vector or the plasmid-encoding SENP1. (E) IRF-1 is highly SUMOylated in ovarian tumors. Western blot analysis was performed by using the indicated antibodies (Left). The star indicates a nonspecific band. RT-PCR results displayed that the amount of IRF-1 mRNA was not significantly changed between the normal cells and the tumor. Ovarian tumor cell lysates were immunopurified with either anti-SUMO-1or control antibody and then incubated with anti-IRF-1 antibody (Right).