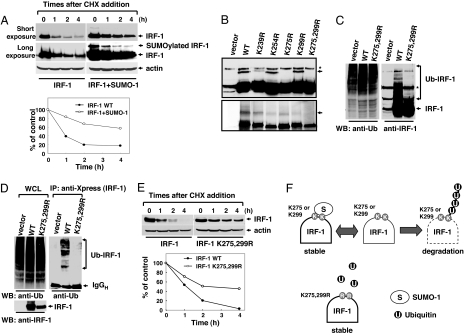
Fig. 2.
IRF-1 is stabilized by SUMOylation. (A) SUMOylated IRF-1 displayed more resistance to protein degradation. HEK293 cells were transiently transfected with IRF-1 in the presence or absence of SUMO-1 and treated with 20 μM cycloheximide for the indicated periods. The stability of IRF-1 alone (filled circles) and IRF-1 with the coexpression of SUMO-1 (open circles) was evaluated under conditions in which synthesis was blocked. (B) Generation of SUMO-deficient IRF-1 mutants. Cells were transfected with IRF-1, IRF-1 K254R, IRF-1 K275R, IRF-1 K299R, and IRF-1 K275,299R with SUMO-1 (Upper). The arrow specifies SUMOylated IRF-1. These constructs were used for the in vitro SUMOylation assay (Lower). The star indicates a nonspecific band. (C) SUMO and ubiquitin target the same lysine residues of IRF-1. HEK293 cells were transiently transfected with IRF-1 or IRF-1 (K275,299R) and treated with 10 μM MG132 for 12 h. Cell lysates were subjected to immunoblotting with antiubiquitin (Left) and anti-IRF-1 antibodies (Right). (D) Cells were transfected with plasmids encoding Xpress-tagged IRF-1 or Xpress-tagged IRF-1 mutant (K275,299R). The proteins were immunoprecipitated with anti-Xpress antibody and then immunoblotted with antiubiquitin antibodies (Right). Whole-cell lysates (WCL) were subjected to immunoblotting with antiubiquitin (Left) and anti-IRF-1 antibodies (Right). (E) The IRF-1 mutant (K275,299R) displayed greater resistance to protein degradation. The stability of wild-type IRF-1 (filled circles) and IRF-1 mutant (K275,299R) (open circles) was evaluated. (F) Schematic model showing antagonistic effects of SUMO and ubiquitin on IRF-1 protein stability.