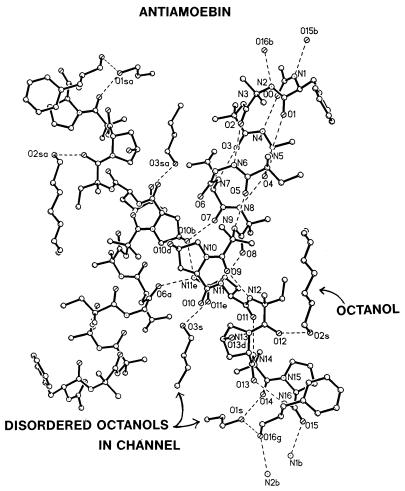
Figure 1.
Conformation of antiamoebin 1 and the channel formed by three symmetry-related peptide molecules. (The third molecule is directly behind one of the molecules shown.) The channel is in the closed position that involves the N(11e)H⋅⋅⋅O(6a) hydrogen bond. An open channel position was also found in the Leu-zervamicin crystal 2. The dashed lines indicate hydrogen bonds. A well-ordered octanol molecule is shown on the concave hydrophobic side of the peptide. A symmetry related octanol is shown on the left. Only a portion of two more octanol molecules that reside in the channel is shown because the remainder of the hydrophobic chains of the octanol molecules are disordered to various degrees.