Abstract
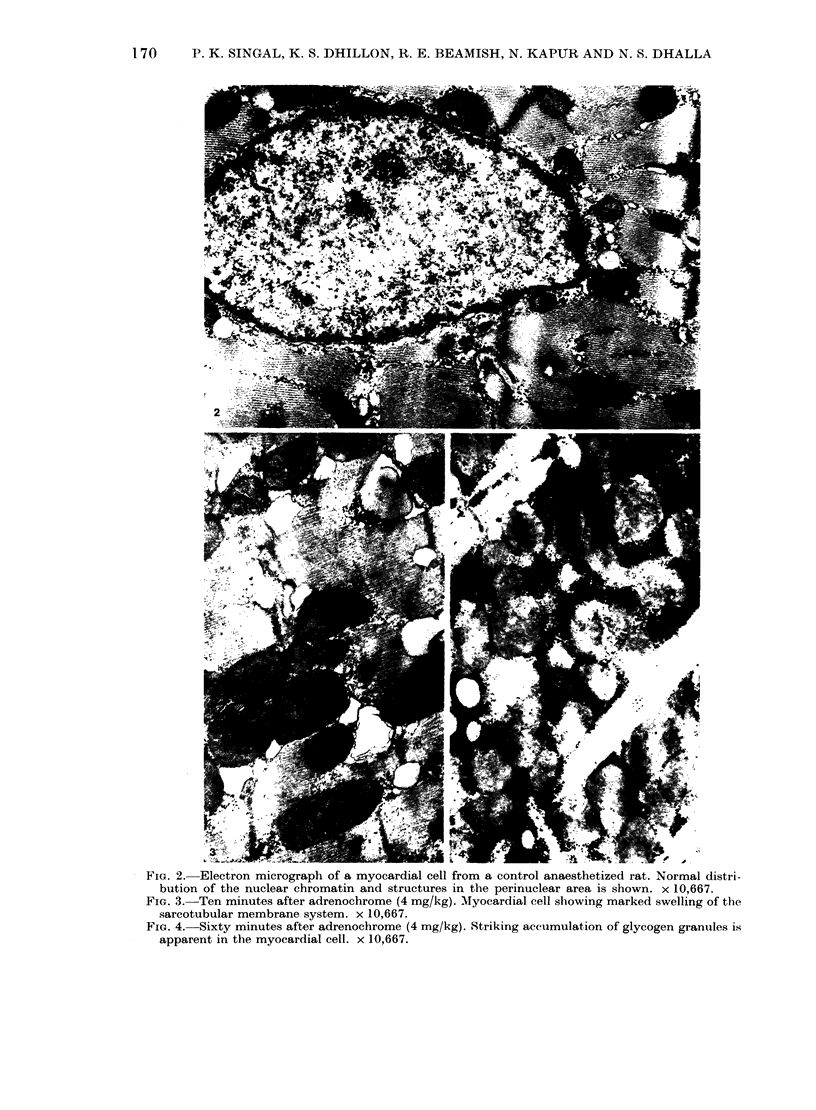
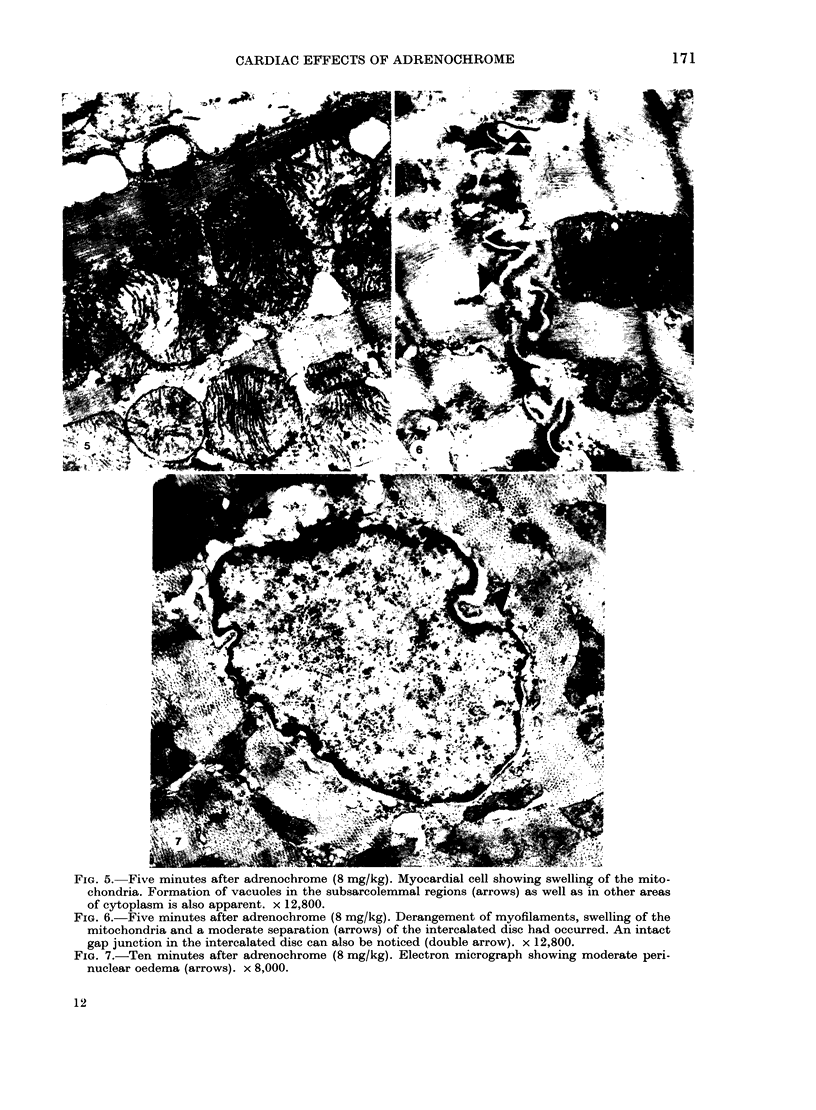
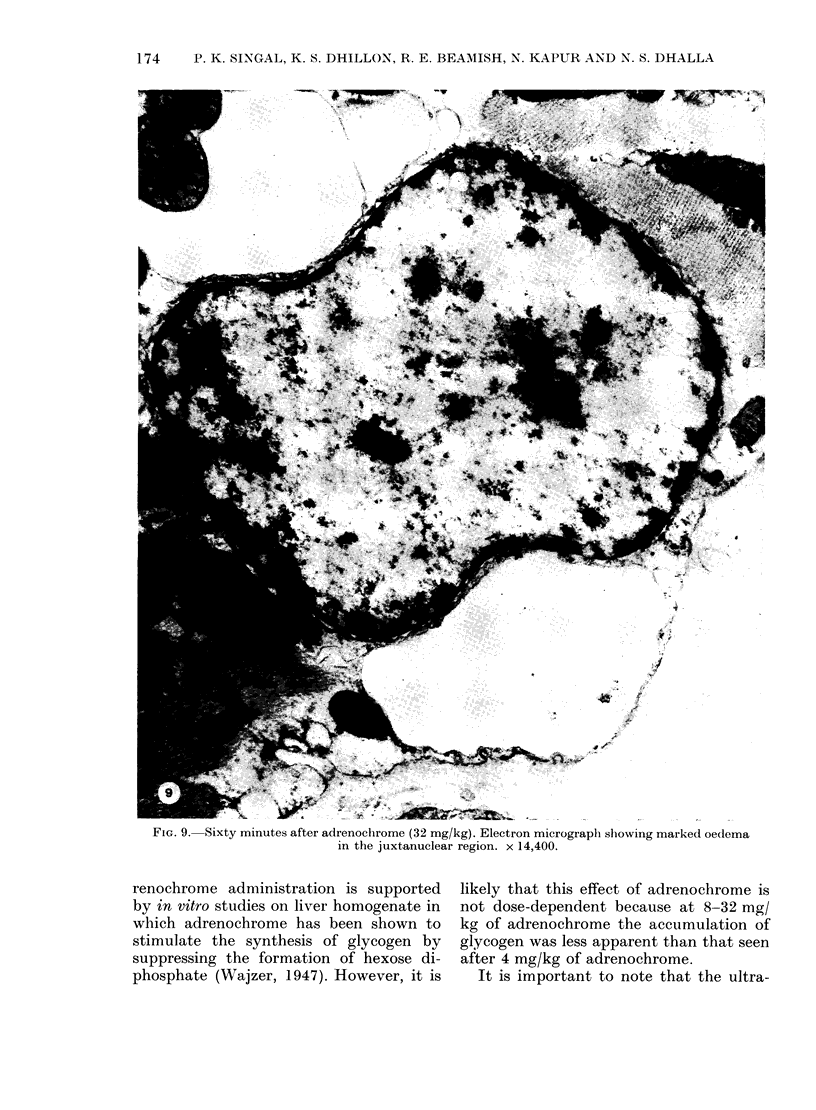
In vivo effects of adrenochrome (1-32 mg/kg), an oxidation product of catecholamines, on the heart ultrastructure, ECG and blood pressure were studied in rats over a period of 60 min following a single i.v. injection of the drug. One milligram of the drug had no influence on the myocardium or the cardiovascular system, whereas maximum changes in these parameters were recorded at 32 mg/kg of adrenochrome. The maximum structural damage, reached within 5-10 min, included marked swelling of mitochondria and sarcotubular system, intracellular and perinuclear oedema, hypercontraction of myofibrils and partial separation of the intercalated disc. Ultrastructural changes in the myocardium due to 4 and 8 mg of adrenochrome were not accompanied by any cardiovascular effects and the changes were fully reversed within 60 min of the injection of the drug. However, at 16 and 32 mg/kg of adrenochrome both heart rate and blood pressure were depressed within 5 min of drug administration. At these concentrations of adrenochrome arrhythmias, mainly due to premature ventricular contractions, were also noticed. Ultrastructural and cardiovascular changes seen at these higher concentrations of adrenochrome showed only a partial recovery. The data indicates that adrenochrome-induced ultrastructural changes in the heart are due to a direct myocardial effect of the drug which may not involve haemodynamic changes and the latter are most probably a consequence of this effect. However, the present study has not been able to rule out direct vascular effects at higher concentrations of adrenochrome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom S., Cancilla P. A. Myocytolysis and mitochondrial calcification in rat myocardium after low doses of isoproterenol. Am J Pathol. 1969 Mar;54(3):373–391. [PMC free article] [PubMed] [Google Scholar]
- Bloom S., Davis D. Isoproterenol myocytolysis and myocardial calcium. Recent Adv Stud Cardiac Struct Metab. 1974;4:581–590. [PubMed] [Google Scholar]
- CHAPPEL C. I., RONA G., BALAZS T., GAUDRY R. Comparison of cardiotoxic actions of certain sympathomimetic amines. Can J Biochem Physiol. 1959 Jan;37(1):35–42. [PubMed] [Google Scholar]
- Carlsten A., Poupa O. Effect of time on the response to cyanide anoxia of frog heart damaged by isoproterenol. Acta Pharmacol Toxicol (Copenh) 1977 Jul;41(1):18–24. doi: 10.1111/j.1600-0773.1977.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Csapó Z., Dusek J., Rona G. Early alterations of the cardiac muscle cells in isoproterenol-induced necrosis. Arch Pathol. 1972 Apr;93(4):356–365. [PubMed] [Google Scholar]
- Dhalla N. S., Yates J. C., Lee S. L., Singh A. Functional and subcellular changes in the isolated rat heart perfused with oxidized isoproterenol. J Mol Cell Cardiol. 1978 Jan;10(1):31–41. doi: 10.1016/0022-2828(78)90004-4. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A., Janke J., Döring H. J., Leder O. Myocardial fiber necrosis due to intracellular Ca overload-a new principle in cardiac pathophysiology. Recent Adv Stud Cardiac Struct Metab. 1974;4:563–580. [PubMed] [Google Scholar]
- HANDFORTH C. P. Isoproterenol-induced myocardial infarction in animals. Arch Pathol. 1962 Feb;73:161–165. [PubMed] [Google Scholar]
- Kjekshus J. K. Role of free fatty acids in catecholamine-induced cardiac necrosis. Recent Adv Stud Cardiac Struct Metab. 1975;6:183–191. [PubMed] [Google Scholar]
- Kutsuna F. Electron microscopic studies on isoproterenol-induced myocardial lesion in rats. Jpn Heart J. 1972 Mar;13(2):168–175. doi: 10.1536/ihj.13.168. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruffo C. A. Fine structural study of myocardial changes induced by isoproterenol in rhesus monkeys. (Macaca mulatta). Am J Pathol. 1967 Jan;50(1):27–37. [PMC free article] [PubMed] [Google Scholar]
- Muir A. R. The effects of divalent cations on the ultrastructure of the perfused rat heart. J Anat. 1967 Apr;101(Pt 2):239–261. [PMC free article] [PubMed] [Google Scholar]
- Ostádal B., Rychterová V., Poupa O. Isoproterenol-induced acute experimental cardiac necrosis in the turtle (Testudo Horsfieldi). Am Heart J. 1968 Nov;76(5):645–649. doi: 10.1016/0002-8703(68)90163-4. [DOI] [PubMed] [Google Scholar]
- ROSENBLUM I., WOHL A., STEIN A. A. STUDIES IN CARDIAC NECROSIS. II. CARDIOVASCULAR EFFECTS OF SYMPATHOMIMETIC AMINES PRODUCING CARDIAC LESIONS. Toxicol Appl Pharmacol. 1965 Jan;7:9–17. doi: 10.1016/0041-008x(65)90068-2. [DOI] [PubMed] [Google Scholar]
- Regan T. J., Markov A., Khan M. I., Jesrani M. U., Oldewurtel H. A., Ettinger P. O. Myocardial ion and lipid changes during ischemia and catecholamine induced necrosis: Relation to regional blood flow. Recent Adv Stud Cardiac Struct Metab. 1972;1:656–664. [PubMed] [Google Scholar]
- SZAKACS J. E., CANNON A. L-Norepinephrine myocarditis. Am J Clin Pathol. 1958 Nov;30(5):425–434. doi: 10.1093/ajcp/30.5.425. [DOI] [PubMed] [Google Scholar]
- Severin E., Sartore S., Schiaffino S. Direct toxic effect of isoproterenol on cultured cardiac muscle cells. Experientia. 1977 Nov 15;33(11):1489–1489. doi: 10.1007/BF01918826. [DOI] [PubMed] [Google Scholar]
- Singal P. K., Dhillon K. S., Beamish R. E., Dhalla N. S. Protective effect of zinc against catecholamine-induced myocardial changes electrocardiographic and ultrastructural studies. Lab Invest. 1981 May;44(5):426–433. [PubMed] [Google Scholar]
- Singal P. K., Yates J. C., Beamish R. E., Dhalla N. S. Influence of reducing agents on adrenochrome-induced changes in the heart. Arch Pathol Lab Med. 1981 Dec;105(12):664–669. [PubMed] [Google Scholar]
- Slater E. C. The measurement of the cytochrome oxidase activity of enzyme preparations. Biochem J. 1949;44(3):305–318. doi: 10.1042/bj0440305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel B., Jequier E., Sjoerdsma A., Lovenberg W. Effect of catecholamines and adrenergic blocking agents on oxidative phosphorylation in rat heart mitochondria. Circ Res. 1966 Dec;19(6):1050–1061. doi: 10.1161/01.res.19.6.1050. [DOI] [PubMed] [Google Scholar]
- Valerino D. M., McCormack J. J. Xanthine oxidase-mediated oxidation of epinephrine. Biochem Pharmacol. 1971 Jan;20(1):47–55. doi: 10.1016/0006-2952(71)90470-9. [DOI] [PubMed] [Google Scholar]
- WADDELL A. W. Adrenaline, noradrenaline and potassium fluxes in rabbit auricles. J Physiol. 1961 Feb;155:209–220. doi: 10.1113/jphysiol.1961.sp006623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. C., Beamish R. E., Dhalla N. S. Ventricular dysfunction and necrosis produced by adrenochrome metabolite of epinephrine: relation to pathogenesis of catecholamine cardiomyopathy. Am Heart J. 1981 Aug;102(2):210–221. doi: 10.1016/s0002-8703(81)80012-9. [DOI] [PubMed] [Google Scholar]
- Yates J. C., Dhalla N. S. Induction of necrosis and failure in the isolated perfused rat heart with oxidized isoproterenol. J Mol Cell Cardiol. 1975 Nov;7(11):807–816. doi: 10.1016/0022-2828(75)90132-7. [DOI] [PubMed] [Google Scholar]
- Yates J. C., Taam G. M., Singal P. K., Beamish R. E., Dhalla N. S. Modification of adrenochrome-induced cardiac contractile failure and cell damage by changes in cation concentrations. Lab Invest. 1980 Oct;43(4):316–326. [PubMed] [Google Scholar]
- Yates J. C., Taam G. M., Singal P. K., Beamish R. E., Dhalla N. S. Protection against adrenochrome-induced myocardial damage by various pharmacological interventions. Br J Exp Pathol. 1980 Jun;61(3):242–255. [PMC free article] [PubMed] [Google Scholar]