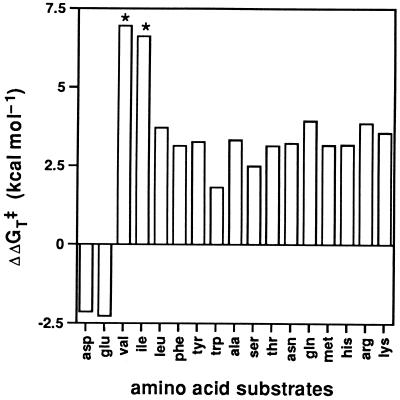
Figure 2.
Stabilization of the activation free energy for various amino acid substrates by AV5A-7, relative to the wild-type AspAT. ΔΔGT‡ = RTln{(kcat/Km)AV5A-7/(kcat/Km)WT}. Substrate amino acids are shown by three-letter abbreviations. Positive bars indicate that the catalytic efficiency of AV5A-7 is higher than that of the wild-type AspAT, and negative bars indicate vice versa. Exact values could not be determined for valine and isoleucine; therefore, ΔΔGT‡ values for their 2-oxo acids are shown for these amino acids (asterisks).