Abstract
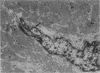
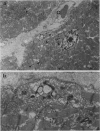
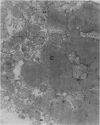
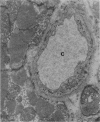
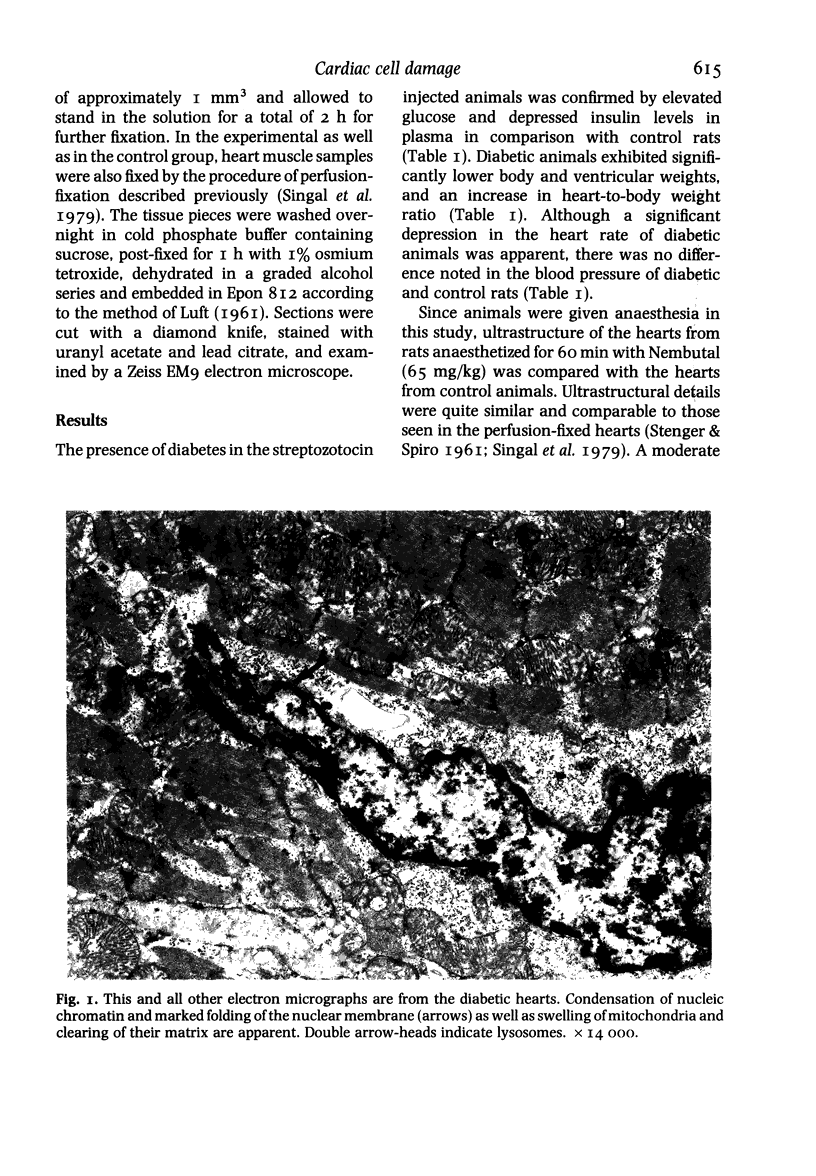
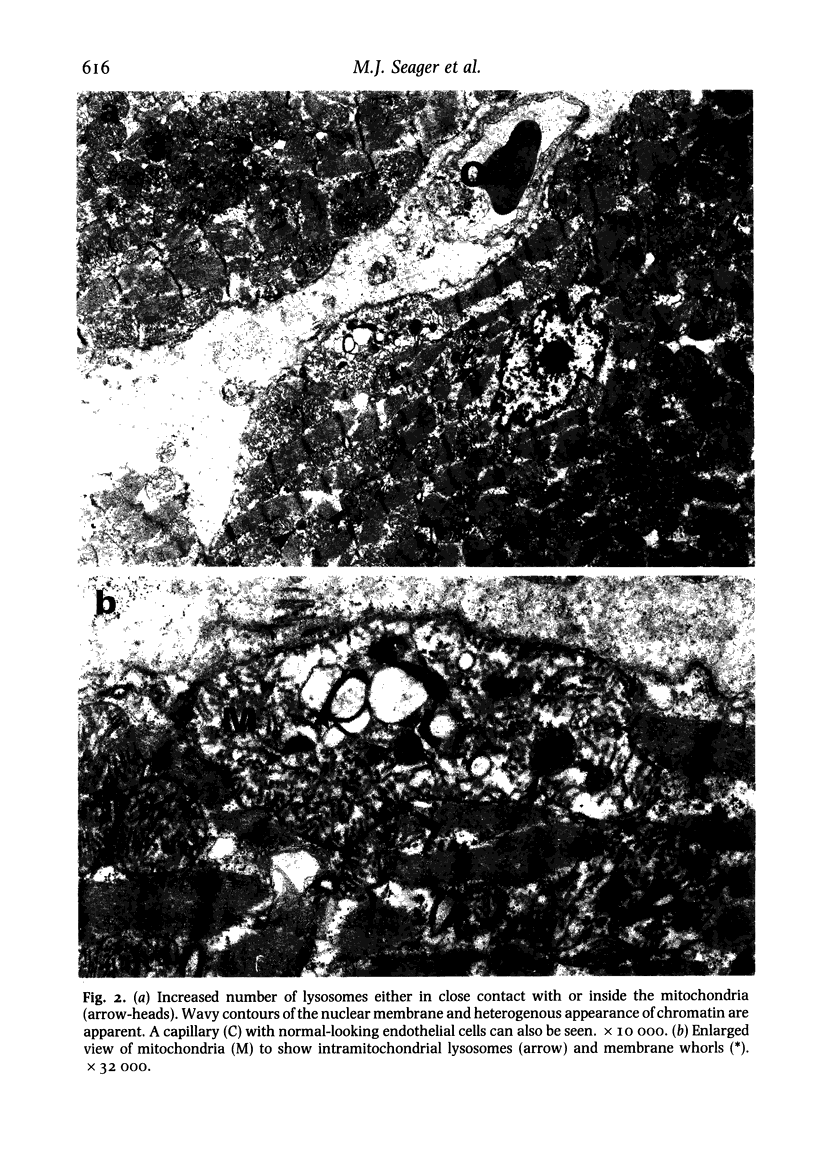
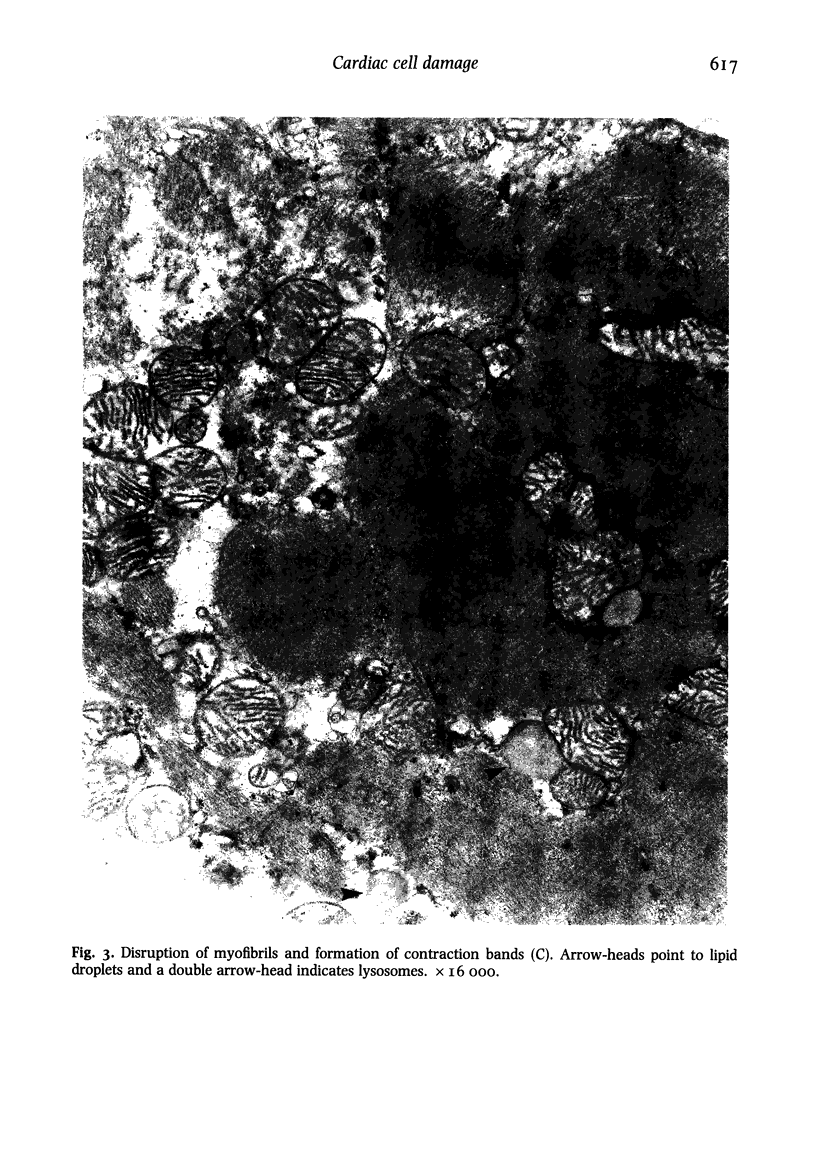
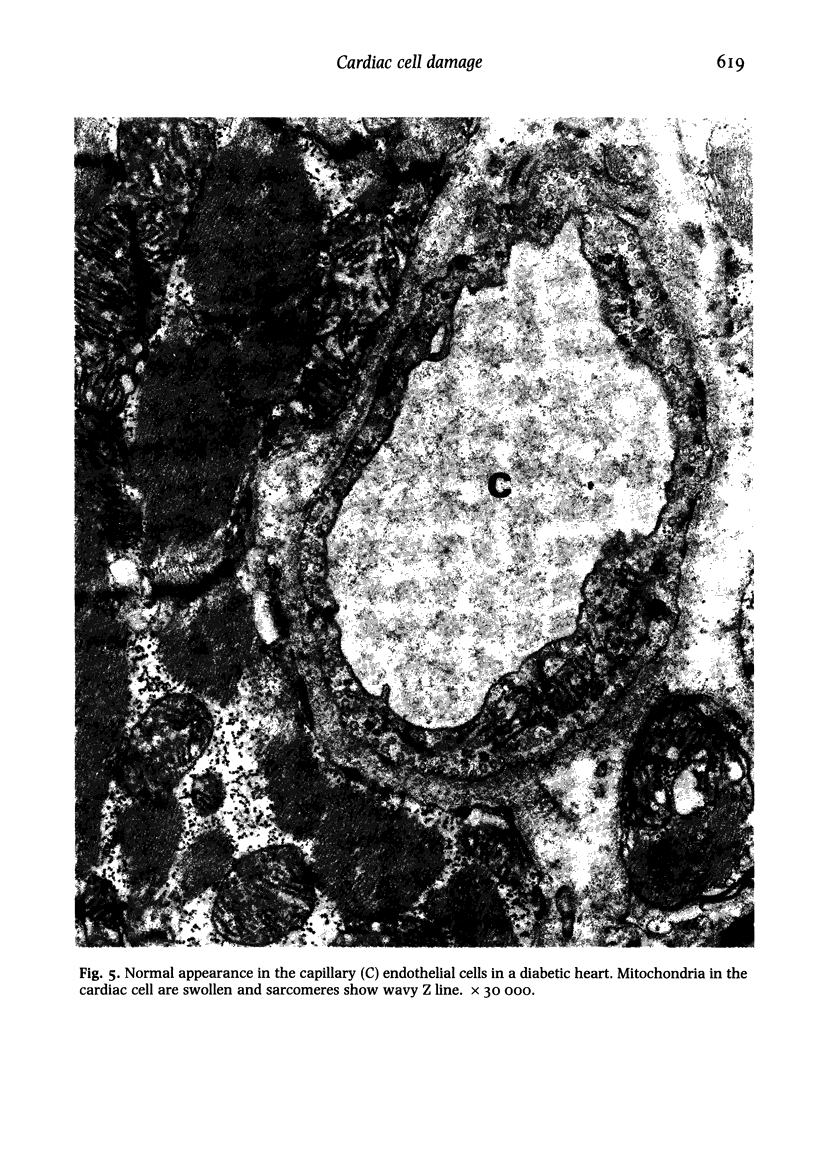
Ultrastructural changes in heart muscle due to chronic diabetes subsequent to a single injection of streptozotocin (65 mg/kg body wt, i.v.) were studied in rats. Presence of diabetes was indicated by hyperglycaemia (plasma glucose, control, 120 +/- 7; diabetic, 448 +/- 21 mg/dl) as well as hypo-insulinaemia (plasma insulin, control, 25.6 +/- 5.2; diabetic, 11.2 +/- 0.5 microU/ml). After 8 weeks of diabetes, the hearts were processed for electron microscopic examination. Cardiac muscle cells in diabetic hearts showed condensation of nuclear chromatin and folding of nuclear membranes. Swelling of mitochondria, clearing of mitochondrial matrix and incorporation of lysosomal membranes into mitochondrial matrix was also noted. A marked increase in both lysosomes and lipid droplets was apparent. Focal areas in diabetic hearts showed contracted sarcomeres, myofibrillar degeneration and separation of the intercalated disc. Atherosclerotic plaque formation as well as structural changes in the smooth muscle or endothelial cells in the small arteries, arterioles or capillaries were not seen to accompany the structural changes in the cardiac muscle cells of the diabetic hearts. This study provides strong evidence for the occurrence of primary myocardial disease in streptozotocin-induced chronic diabetes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal M. K. Streptozotocin: mechanisms of action: proceedings of a workshop held on 21 June 1980, Washington, DC. FEBS Lett. 1980 Oct 20;120(1):1–3. doi: 10.1016/0014-5793(80)81031-3. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Pierce G. N., Panagia V., Singal P. K., Beamish R. E. Calcium movements in relation to heart function. Basic Res Cardiol. 1982 Mar-Apr;77(2):117–139. doi: 10.1007/BF01908167. [DOI] [PubMed] [Google Scholar]
- Fein F. S., Kornstein L. B., Strobeck J. E., Capasso J. M., Sonnenblick E. H. Altered myocardial mechanics in diabetic rats. Circ Res. 1980 Dec;47(6):922–933. doi: 10.1161/01.res.47.6.922. [DOI] [PubMed] [Google Scholar]
- Ganguly P. K., Pierce G. N., Dhalla K. S., Dhalla N. S. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol. 1983 Jun;244(6):E528–E535. doi: 10.1152/ajpendo.1983.244.6.E528. [DOI] [PubMed] [Google Scholar]
- Giacomelli F., Wiener J. Primary myocardial disease in the diabetic mouse. An ultrastructural study. Lab Invest. 1979 Apr;40(4):460–473. [PubMed] [Google Scholar]
- Hamby R. I., Zoneraich S., Sherman L. Diabetic cardiomyopathy. JAMA. 1974 Sep 23;229(13):1749–1754. [PubMed] [Google Scholar]
- Hoftiezer V., Carpenter A. M. Comparison of streptozotocin and alloxan-induced diabetes in the rat, including volumetric quantitation of the pancreatic islets. Diabetologia. 1973 Jun;9(3):178–184. doi: 10.1007/BF01219780. [DOI] [PubMed] [Google Scholar]
- Hug G., Schubert W. K. Idiopathic cardiomyopathy. Mitochondrial and cytoplasmic alterations in heart and liver. Lab Invest. 1970 Jun;22(6):541–552. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledet T. Histological and histochemical changes in the coronary arteries of old diabetic patients. Diabetologia. 1968 Nov;4(5):268–272. doi: 10.1007/BF01309899. [DOI] [PubMed] [Google Scholar]
- Penpargkul S., Schaible T., Yipintsoi T., Scheuer J. The effect of diabetes on performance and metabolism of rat hearts. Circ Res. 1980 Dec;47(6):911–921. doi: 10.1161/01.res.47.6.911. [DOI] [PubMed] [Google Scholar]
- Pierce G. N., Dhalla N. S. Sarcolemmal Na+-K+-ATPase activity in diabetic rat heart. Am J Physiol. 1983 Sep;245(3):C241–C247. doi: 10.1152/ajpcell.1983.245.3.C241. [DOI] [PubMed] [Google Scholar]
- Pierce G. N., Kutryk M. J., Dhalla N. S. Alterations in Ca2+ binding by and composition of the cardiac sarcolemmal membrane in chronic diabetes. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5412–5416. doi: 10.1073/pnas.80.17.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal P. K., Matsukubo M. P., Dhalla N. S. Calcium-related changes in the ultrastructure of mammalian myocardium. Br J Exp Pathol. 1979 Feb;60(1):96–106. [PMC free article] [PubMed] [Google Scholar]
- Sulkin N. M., Sulkin D. F. An electron microscopic study of the effects of chronic hypoxia on cardiac muscle, hepatic, and autonomic ganglion cells. Lab Invest. 1965 Aug;14(8):1523–1546. [PubMed] [Google Scholar]
- Vihert A. M., Zhdanov V. S., Matova E. E. Atherosclerosis of the aorta and coronary vessels of the heart in cases of various diseases. J Atheroscler Res. 1969 Mar-Apr;9(2):179–192. doi: 10.1016/s0368-1319(69)80053-0. [DOI] [PubMed] [Google Scholar]
- WHEAT M. W., Jr ULTRASTRUCTURE AUTORADIOGRAPHY AND LYSOSOME STUDIES IN MYOCARDIUM. J Mt Sinai Hosp N Y. 1965 Mar-Apr;32:107–121. [PubMed] [Google Scholar]