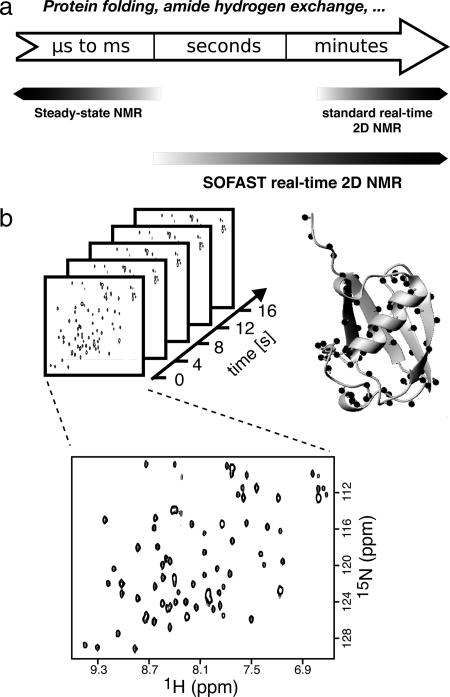
Fig. 1.
SOFAST real-time 2D NMR closes the seconds time gap for NMR studies of protein dynamics. (a) Time scales of biophysical processes such as protein folding or amide hydrogen exchange, and NMR methods available to study the kinetics of these processes at atomic resolution. (b) Principle of SOFAST real-time 2D NMR. A series of 2D FTA-SOFAST-HMQC spectra is recorded after initiating a kinetic change in the protein state. Each cross-peak reports on the local structure and dynamics at the site of a single amide group. The bottom spectrum has been recorded in an experimental time of 4 s on a 0.2 mM 15N-labeled sample of ubiquitin at a magnetic field strength of 18.8 T by using a cryogenically cooled probe.