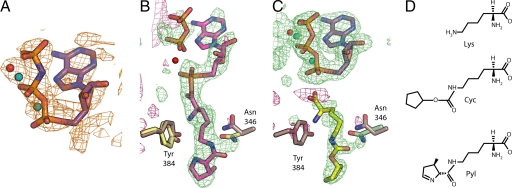
Fig. 1.
PylRS substrates. (A) The solvent-flattened, experimentally phased map calculated to 2.5 Å resolution and contoured at 2σ for the PylRS structure bound to AMP–PNP. The final refined model of AMP–PNP is shown as sticks and the magnesium ions as blue spheres. (B and C) Unbiased Fo-Fc maps calculated with experimental amplitudes from data collected from PylRS crystals that were soaked with either ATP and Pyl to 2.2 Å resolution (B) or ATP and Cyc to 1.9 Å (C). Calculated amplitudes were generated from a model of PylRS that lacked nucleotide. Both maps are contoured at +1.5σ (green) and −1.5σ (maroon). The position of the side chains from the original AMP–PNP complex are shown in brown, whereas the final refined positions of the side chains for the complex with Pyl–AMP are in yellow (B) or are displayed in green for the complex with Cyc (C). The final positions of the Pyl–AMP and pyrophosphate are in pink (B), and the amino acid substrate Cyc is shown in yellow (C). The final positions of two magnesium ions (blue spheres) were confirmed by anomalous difference maps from crystals that had been soaked with manganese. Based on similarity with LysRS (19), a third metal position (red sphere) was identified, but tentatively modeled as a water due to a lack of anomalous difference density. (D) Chemical diagrams of Lys, Cyc, and Pyl.