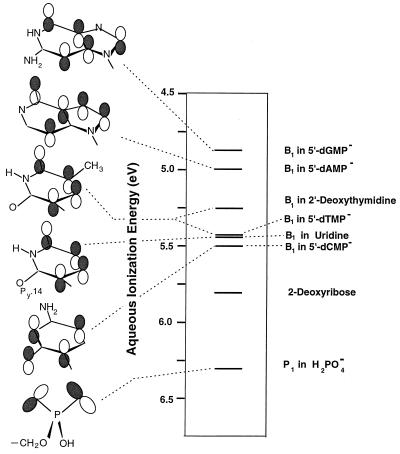
Figure 6.
Aqueous base (B1) ionization energies and orbital diagrams for 5′-dGMP−, 5′-dAMP−, 5′-dCMP−, 5′-dTMP−, 2′-deoxythymidine and uridine. The small difference between the ionization energies of 5′-dCMP− and 5′-dTMP− makes the ordering uncertain; however, in the gas phase, the B1 ionization potential of the model compound 1-MeC is less than that of 1-MeT. Results are also given for 2-deoxyribose and H2PO4−. All ionization energies were obtained from Eq. 1. For H2PO4−, the gas-phase P1 ionization potential was calculated at the MP2/6–31+G* level (ref. 31), and ΔΔGHYD was evaluated by using H2PO4− and H2PO4• charge distributions from 3–21G SCF calculations. For 5′-dGMP− and 5′-dAMP−, uncorrected B1 gas-phase IPs were obtained from 3–21G SCF calculations, and values of ΔΔGHYD were the same as those used in earlier investigations of nucleotide electron donating properties (see refs. 13 and 17). For 2′-deoxythymidine, uridine, and 2-deoxyribose, aqueous ionization energies were obtained with the same method used to obtain 5′-dCMP− and 5′-dTMP− ionization energies. 3-OH-THF was used as a model compound to correct the gas-phase IP of 2-deoxyribose. 1,9-Dimethylguanine, 9-methyladenine, and 1-methyluracil with experimental adiabatic IPs of 7.71, 7.97, and 8.75 eV, respectively, were used as model compounds to correct the gas-phase B1 ionization potentials of 5′-dGMP−, 5′-dAMP−, and uridine, respectively (see refs. 11, 13, 17, and 30).