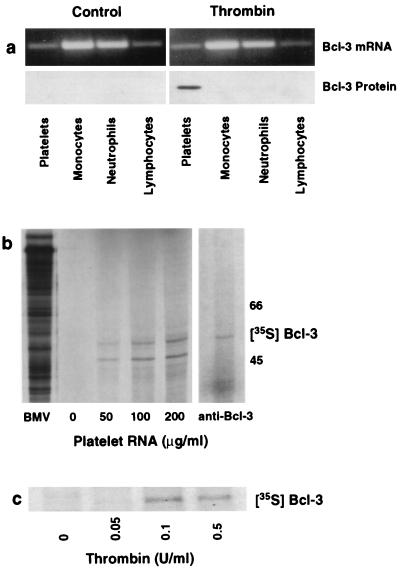
Figure 2.
Platelets synthesize Bcl-3 protein from preexisting mRNA. (a) Thrombin stimulates Bcl-3 protein expression in platelets but not in other blood cells. Platelets and leukocyte subpopulations were isolated, each cell population was exposed to thrombin (0.1 unit/ml) for 2 hr, and total RNA and protein were isolated for reverse transcription–PCR (Upper) and Western blot analysis (Lower). We found the same result in three experiments. (b) In vitro translation of total RNA from platelets results in Bcl-3 protein synthesis. (Left) Increasing concentrations of platelet RNA (μg/ml) were translated in vitro in the presence of [35S]methionine and subsequently examined by autoradiography. mRNA from Brome mosaic virus (BMV) served as a positive control. (Right) The [35S]methionine-labeled protein from platelet RNA translated in vitro was immunoprecipitated with an anti-Bcl-3 antibody as described in Materials and Methods. A second experiment yielded the same result. (c) Radiolabeled incorporation of [35S]methionine into Bcl-3 in platelets activated with thrombin. Platelets were preincubated with [35S]methionine and stimulated with increasing concentrations of thrombin, and lysates were incubated with an anti-Bcl-3 antibody after 4 hr. This figure is representative of three experiments.