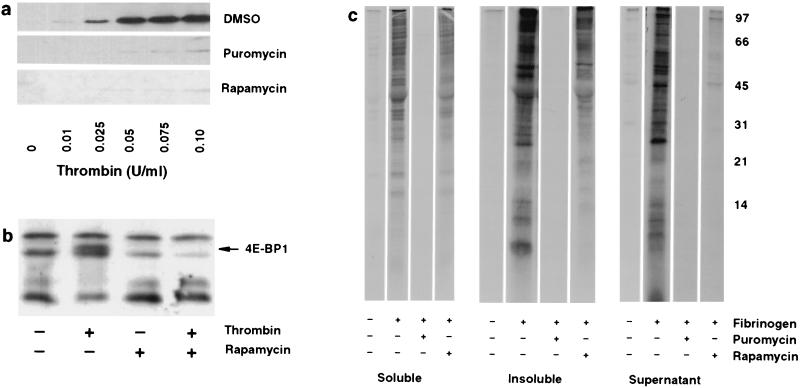
Figure 3.
Synthesis of Bcl-3 and other proteins is regulated by rapamycin in signaled platelets. (a) Inhibition of translation markedly attenuates Bcl-3 expression in platelets signaled by thrombin. Platelets were pretreated for 2 hr with dimethyl sulfoxide, puromycin, or rapamycin and activated with increasing concentrations of thrombin for 2 hr, and newly synthesized Bcl-3 was identified by Western blot analysis. (b) Rapamycin inhibits 4E-BP1 phosphorylation in activated platelets. Platelets were pretreated with rapamycin, and 4E-BP1, a protein that migrates in SDS/PAGE as multiple species (20–24 kDa), was identified by Western blot analysis 15 min after stimulation with thrombin (0.01 unit/ml). The solid arrow indicates a phosphorylated form of 4E-BP1 as described by Lin and Lawrence (11). a and b are representative of five experiments. (c) Platelets stimulated by adhesion to fibrinogen synthesize proteins. Platelets were pretreated with dimethyl sulfoxide, puromycin, or rapamycin. After pretreatment, they were incubated with [35S]methionine and allowed to adhere to immobilized fibrinogen for 8 hr. Supernatants were removed and the platelets were washed extensively with M199 buffer containing methionine, lysed, and fractionated into soluble and insoluble portions, and the labeled proteins were separated in a 15% SDS-polyacrylamide gel. This experiment is representative of three others.