Figure 3. Comparison of NBD conformations in Sse1 and Hsp70s.
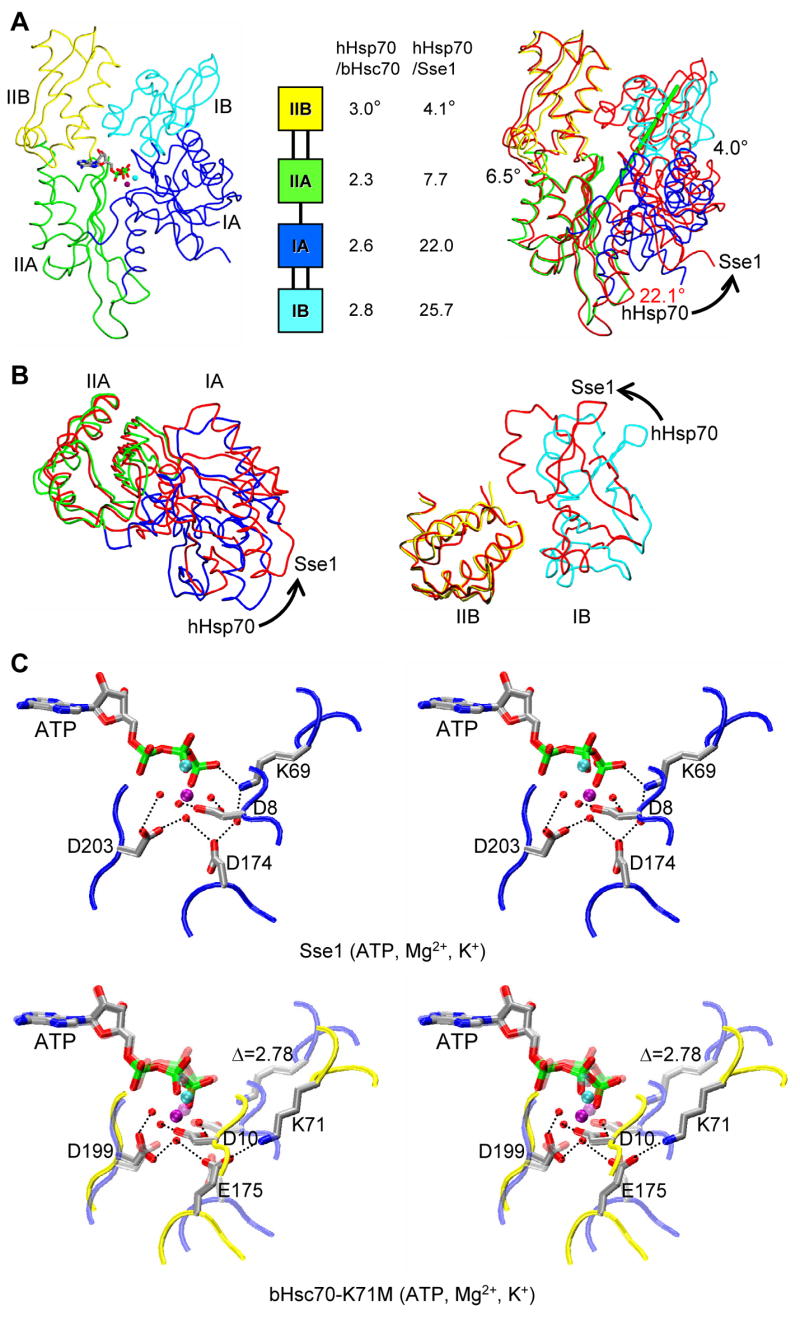
(A) Cα traces of Sse1 (left) and Sse1 superimposed onto hHsp70 (right, based on Cαs in subdomain IIA), and relative domain orientations (middle, based on superpositions of adenine-ribose-Pα atoms). NBDs are oriented in the canonical, front-face view. Sse1 subdomains (left) and hHsp70 subdomains (right) are colored: IA (blue), IB (cyan), IIA (green) and IIB (yellow). Sse1 (right) is red. ATP is drawn and colored as in 3C. Rotation relating IA and IIA is about the green rod (right). (B) Superpositions of Sse1 and hHsp70 NBD subdomains viewed from above A, rotated 90° around the horizontal. Coloring is as in A (right). Superpositions of both IA/IIA (left) and IB/IIB (right) are based on Cαs in lobe II subdomains. (C) Stereodrawing of ATP catalytic sites in Sse1 (above) and bHsc70 (below). The view is changed slightly from 3A. Adenine-ribose-Pα atoms are superimposed. The image for bHsc70(ATP) is a composite from bHsc70(ATP, K71M) (PDB 1KAX) and side-chain atoms of Lys71 translated into Cα superposition from bHsc70(ATP, D199N) (PDB 1NGF); a subdued version of Sse1 is superimposed. Side chains are shown for four residues shown for bHsc70 to be most critical for ATPase function and for counterparts in Sse1. Coloring for ATP, ions and side chains has the pattern: carbon (grey), nitrogen (blue), oxygen (red), phosphorous (green), Mg2+ (purple) and K+ (cyan); backbone traces are bHsc70 (yellow) and Sse1 (red). Hydrogen bonds are drawn as black dotted lines.
