Figure 3.
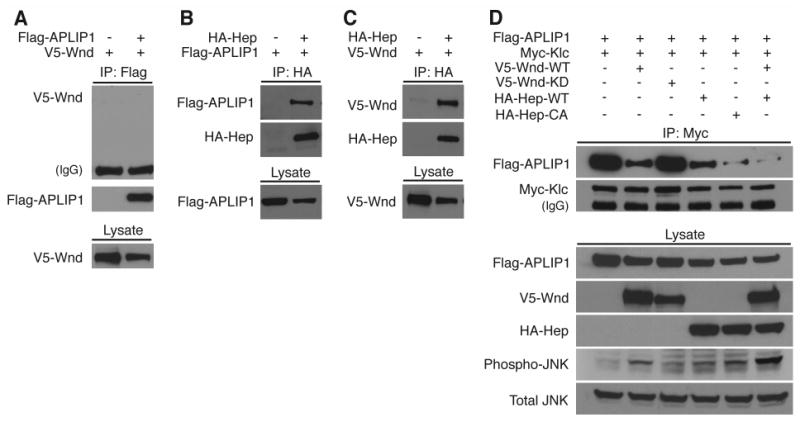
Wnd (MAPKKK) and Hep (MAPKK) associate and their activities inhibit APLIP1 (JIP1)-Klc binding.
Drosophila S2 cells were used for transfection and expression of epitope-tagged constructs as noted across the top of each panel. The presence of proteins was detected in cell lysates (Lysate) or in immunoprecipitation pellets (IP) by western blotting. Proteins detected with specific antibodies are noted to the left of each blot panel. (A) Co-precipitation of Wnd (V5-Wnd) was not detected with APLIP1 (FLAG-APLIP1). (B) APLIP1 co-precipitated with Hep (HA-Hep). (C) Wnd co-precipitated with Hep. (D) APLIP1 co-precipitation with kinesin-1 light chain (Myc-Klc) was partially inhibited by expression of Wnd (V5-Wnd), but not by a kinase-dead mutant Wnd (V5-Wnd-KD). Co-precipitation of APLIP1 with Klc was partially inhibited by expression of wild-type Hep (HA-Hep), and was more completely inhibited by a constitutively active mutant Hep (HA-Hep-CA). Near-complete inhibition was also observed with co-expression of wild-type Wnd and wild-type Hep. Wnd-Hep kinase activity increased the phosphorylation level of endogenous Bsk (compare Total JNK with Phospho-JNK).
