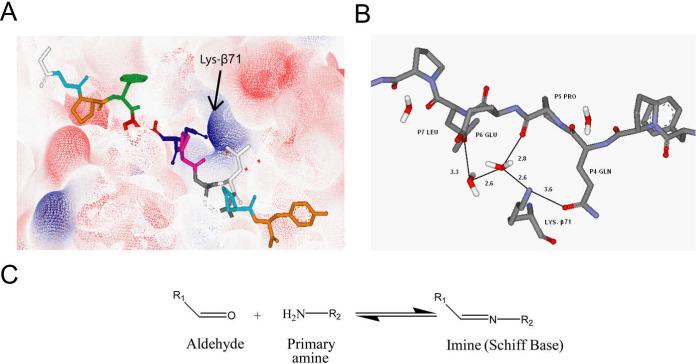
Figure 1.
Rationale behind the design of aldehyde containing gluten peptide analogues. (A) Electron density map showing the positively charged Lys-β71 in the HLA-DQ2 binding groove. (B) Lys-β71 directly forms hydrogen bonds with the P4 glutamine of the DQ2-αI peptide and forms a hydrogen bonding network to interact with the DQ2-αI P6 glutamate (numbers show the distance between corresponding atoms in Å). (C) Aldehydes react with primary amines in a reversible manner to create covalent imine bonds.