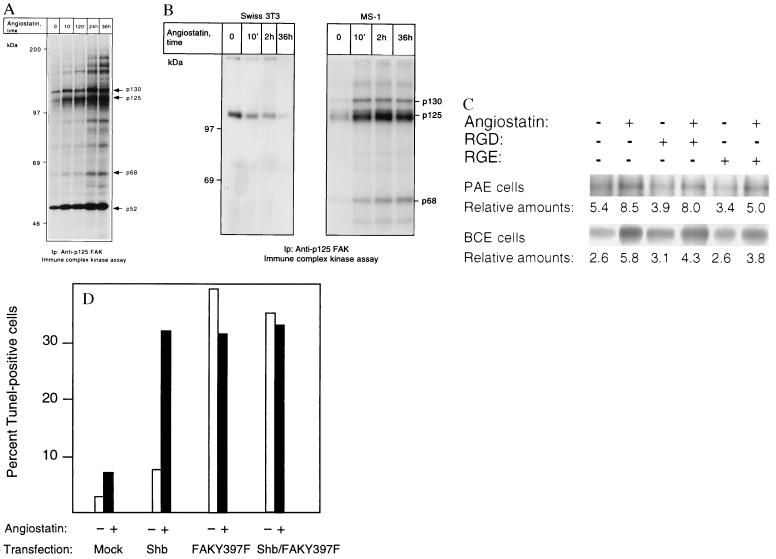
Figure 5.
(A and B) p125 FAK is activated in response to angiostatin treatment only in endothelial cells. PAE/FGFR-1 cells (A) and Swiss 3T3 cells and murine pancreas endothelial cells (MS-1; B) were treated as indicated with 2.5 μg/ml of angiostatin for different time periods and processed for immunoprecipitation by using a mAb against p125 FAK. The immobilized immunoprecipitates were subjected to in vitro kinase assays in the presence of [γ-32P]ATP and analyzed by SDS/PAGE. The migration of 130-, 125-, and 68-kDa components that could correspond to Cas, FAK, and paxillin, respectively, as well as other angiostatin-induced phosphorylated components are indicated by arrows, and the relative masses of marker proteins run in parallel are indicated to the left. (C) Treatment with 1 mM RGD-containing peptide fails to block angiostatin-induced FAK activation. PAE/FGFR-1 cells or BCE cells were treated for 10 min with 2.5 μg/ml of angiostatin in the presence and absence of 1 mM peptide containing the RGD motif (Gly-Arg-Gly-Asp-Thr-Pro; Bachem) or with a control peptide containing RGE instead of RGD. The cells were lysed and processed for immunoprecipitation with anti-FAK mAb, followed by in vitro kinase assay. (D) Effects of angiostatin and dominant-negative FAK-Y397F on BCE cell survival. BCE cells were transfected by using the Lipofectamine procedure using lipofectamine alone (mock), Shb cDNA in pcDNA1, and FAK-Y397F in pCMV (a kind gift from J. T. Parsons, University of Virginia) as indicated. The transfected cells were cultured on coverslips in the absence or presence of 2.5 μg/ml angiostatin for 3 days, after which the cells were processed for TUNEL staining and the percentage of stained nuclei were counted.