Abstract
Phytophthora alni subsp. alni, P. alni subsp. multiformis, and P. alni subsp. uniformis are responsible for alder disease in Europe. Class I and II elicitin gene patterns of P. alni subsp. alni, P. alni subsp. multiformis, P. alni subsp. uniformis, and the phylogenetically close species P. cambivora and P. fragariae were studied through mRNA sequencing and 3′ untranslated region (3′UTR)-specific PCRs and sequencing. The occurrence of multiple 3′UTR sequences in association with identical elicitin-encoding sequences in P. alni subsp. alni indicated duplication/recombination events. The mRNA pattern displayed by P. alni subsp. alni demonstrated that elicitin genes from all the parental genomes are actually expressed in this allopolyploid taxon. The complementary elicitin patterns resolved confirmed the possible involvement of P. alni subsp. multiformis and P. alni subsp. uniformis in the genesis of the hybrid species P. alni subsp. alni. The occurrence of multiple and common elicitin gene sequences throughout P. cambivora, P. fragariae, and P. alni sensu lato, not observed in other Phytophthora species, suggests that duplication of these genes occurred before the radiation of these species.
The oomycete Phytophthora alni (stramenopile lineage) is a recently described highly aggressive pathogen specific to alder trees (Alnus spp.) that is spreading all over Europe, especially along rivers (13). P. alni sensu lato comprises three related taxa: P. alni subsp. alni, P. alni subsp. uniformis, and P. alni subsp. multiformis (5). P. alni subsp. multiformis and P. alni subsp. uniformis are scarce in comparison to P. alni subsp. alni (2, 15). Up to now, all three sibling taxa have been exclusively isolated from alder trees, but P. alni subsp. multiformis and P. alni subsp. uniformis are reported to be significantly less aggressive to Alnus than P. alni subsp. alni (4, 30). The three taxa are phylogenetically close to P. cambivora and P. fragariae, two species that, it was previously suggested, may be P. alni subsp. alni's progenitors (3). However, it was recently inferred from nuclear and mitochondrial gene genealogies (15) and microsatellite patterns (16) that only P. alni subsp. alni is a genuine hybrid taxon, originating from hybridization between P. alni subsp. uniformis and P. alni subsp. multiformis. The status of P. alni subsp. multiformis is still questionable, while P. alni subsp. uniformis's genetic features do not fit with a hybrid origin (15, 16). Up to now, reports of other natural hybrids within the genus Phytophthora are scarce and remain confined to limited geographical areas (19, 20), whereas the allopolyploid taxon P. alni subsp. alni is currently thriving throughout Europe. Since this hybrid taxon is significantly more aggressive than its putative parents, P. alni subsp. multiformis and P. alni subsp. uniformis (4), heterosis or genetic rearrangement may be put forward as explanations for this kind of ecological advantage. Except for the observation of polymorphism in the internal transcribed spacer sequence (3), the extent of genetic rearrangement in the allopolyploid taxon P. alni subsp. alni remains unknown, and the expression of distinct genomes within this hybrid taxon has not been investigated yet.
In order to address these issues, multigenic families, such as elicitin genes, should be of great interest since they are particularly prone to duplication and recombination. Elicitin proteins are restricted to the oomycete genus Phytophthora and a few Pythium species (18, 25). They comprise a large family of polypeptides whose intrinsic function remains largely unknown and that can be divided into at least eight classes (18, 23). Elicitin genes are highly transcribed during vegetative growth, as deduced from their representation in a collection of expressed sequence tags from the broad-host-range pathogen Phytophthora parasitica (23). In particular, group I elicitins (ELI-1), characterized by the typical 98-amino-acid elicitin domain, were reported to be the most abundant secreted proteins in culture filtrates (18). Although elicitin genes are not appropriate for phylogenetic studies because of their multigenic family feature, they can be used as a tool for identification purposes since the amino acid sequence of a given elicitin may provide a signature at the species level (25). In addition, the 3′ untranslated regions (3′UTRs) of class I elicitin genes are strictly conserved within an individual species but diverge between species to such an extent that sequence alignment is almost impossible (1, 6, 11; Panabières et al., unpublished results).
The aims of the present work were, by taking advantage of the high expression of elicitin genes in the Phytophthora genome, (i) to test if the multiple genomes present in the allopolyploid hybrid P. alni subsp. alni are coexpressed or not and (ii) to assess the occurrence of genetic rearrangements. We studied the occurrence and the distribution of members of class I and II elicitin genes and their expression among the different P. alni taxa. This study included the phylogenetically close species P. cambivora, P. fragariae var. fragariae, and P. fragariae var. rubi, previously suggested as P. alni subsp. alni's progenitors (3), for comparison of their elicitin gene patterns.
MATERIALS AND METHODS
Phytophthora isolates and culture.
French isolates of Phytophthora alni sensu lato and other Phytophthora spp. were collected on naturally infected tissues and isolated on PARBHY medium (28). Foreign isolates of P. alni sensu lato and Phytophthora spp. were obtained from the Centraalbureau voor Schimmelcultures (Utrecht, The Netherlands) or from collaborative researchers (Table 1). All the cultures were kept at 10°C in the dark on V8-agar slants (21) and as small V8-agar blocks flooded with sterile distilled water. Five isolates of P. alni subsp. alni (PAA129, PAA130, PAA143, PAA151, and PAA162), three isolates of P. alni subsp. uniformis (PAU60, PAU84, and PAU89), three isolates of P. alni subsp. multiformis (PAM54, PAM71, and PAM73), two isolates of P. cambivora (PC643 and PCjc17), one isolate of P. fragariae var. fragariae (PFF309), and one isolate of P. fragariae var. rubi (PFR109), selected from different geographical locations, were used for in vitro elicitin production and mRNA studies (Table 1).
TABLE 1.
List of the Phytophthora spp. and Pythium spp. used in this study
| Taxon | Isolateg | Host | Origin | Yr | Isolator/supplier |
|---|---|---|---|---|---|
| P. alni subsp. alni | PAA2 | Alnus glutinosa | France | 2002 | J. C. Streito (2N0685) |
| PAA20 | Alnus glutinosa | France | 1997 | J. C. Streito (71T1) | |
| PAA21 | Alnus glutinosa | France | 1997 | J. C. Streito (77T4) | |
| PAA23 | Alnus glutinosa | France | 1997 | J. C. Streito (82T1A) | |
| PAA24 | Alnus glutinosa | France | 1997 | J. C. Streito (84T2) | |
| PAA34 | Alnus glutinosa | France | 1998 | J. C. Streito (98-7-5) | |
| PAA35 | Alnus glutinosa | France | 1998 | J. C. Streito (98-7-6) | |
| PAA38 | Alnus glutinosa | France | 2002 | J. C. Streito (2N0529) | |
| PAA44 | Alnus glutinosa | France | 1998 | J. C. Streito (DSFO98172) | |
| PAA47 | Alnus glutinosa | France | 1999 | J. C. Streito (AUL026/1) | |
| PAA52 | Alnus glutinosa | France | 1999 | J. C. Streito (9900783.4) | |
| PAA53 | Alnus glutinosa | France | 2001 | J. C. Streito (1R0152) | |
| PAA58 | Alnus glutinosa | France | 2001 | J. C. Streito (1N0201) | |
| PAA100 | Alnus glutinosa | France | 2003 | R. Ioos (P1bisa) | |
| PAA103 | Alnus glutinosa | France | 2003 | R. Ioos (P3a) | |
| PAA107 | Alnus glutinosa | France | 2003 | R. Ioos (Priva) | |
| PAA108 | Alnus glutinosa | France | 2003 | R. Ioos (Privb) | |
| PAA109 | Alnus glutinosa | France | 2003 | R. Ioos (P6-2) | |
| PAA110 | Alnus glutinosa | France | 2003 | R. Ioos (P6-1) | |
| PAA111 | A. glutinosa soil | France | 2003 | C. Husson (Ainvelle Sol) | |
| PAA112 | Alnus glutinosa | France | 2003 | C. Husson (2ALD03) | |
| PAA113 | Alnus glutinosa | France | 2003 | C. Husson (102-1) | |
| PAA114 | Alnus glutinosa | France | 2002 | C. Husson (Moselle) | |
| PAA115 | Alnus glutinosa | France | 2002 | C. Husson (370-2) | |
| PAA116 | Alnus glutinosa | France | 2003 | R. Ioos (3N10094-5a) | |
| PAA118 | Alnus glutinosa | France | 2003 | R. Ioos (3N10094-5c) | |
| PAA120 | Alnus glutinosa | France | 2003 | R. Ioos (3N10048-3a) | |
| PAA121 | Alnus glutinosa | France | 2003 | R. Ioos (3N10048-3b) | |
| PAA125 | Alnus glutinosa | France | 2003 | R. Ioos (3N10048-3f) | |
| PAA126 | Alnus glutinosa | France | 2003 | C. Husson (Ainvelle4-4) | |
| PAA127 | Alnus glutinosa | France | 2003 | C. Husson (Ainvelle1-2) | |
| PAA128 | Alnus glutinosa | France | 2003 | C. Husson (Ainvelle1-1) | |
| PAA129* | Alnus glutinosa | France | 2003 | G. Capron (703) | |
| PAA130* | Alnus glutinosa | France | 2003 | R. Ioos (1429-6b) | |
| PAA131 | A. glutinosa, soil | France | 2003 | C. Husson (Sol A15) | |
| PAA132 | A. glutinosa, soil | France | 2003 | C. Husson (Sol A1) | |
| PAA133 | A. glutinosa, soil | France | 2003 | C. Husson (Sol A7) | |
| PAA151* | Alnus glutinosa | France | 2004 | B. Thoirain (2051000-D12) | |
| PAA185 | Alnus glutinosa | France | 2004 | R. Ioos (4N1605) | |
| PAA29 | Alnus glutinosa | Belgium | 1999 | J. C. Streito (9900715.6) | |
| PAA86 | Alnus glutinosa | Belgium | 1999 | D. De Merlier (2198c) | |
| PAA88 | Alnus glutinosa | Belgium | 2001 | D. De Merlier (2295c) | |
| PAA70 | Alnus sp. | The Netherlands | Unknown | W. Man in't Veld (PD2010953) | |
| PAA74 | Alnus glutinosa | Scotland | 2000 | G. Mackaskill (P1275) | |
| PAA75 | Alnus viridis | Scotland | 2000 | J. Gibbs (P1272) | |
| PAA76 | Alnus glutinosa | Scotland | 2000 | J. Gibbs (P1271) | |
| PAA77 | Alnus glutinosa | Scotland | 2000 | J. Delcan (P1270) | |
| PAA78 | Alnus glutinosa | England | 1997 | J. Delcan (P1960) | |
| PAA79 | Alnus glutinosa | England | 1997 | J. Delcan (P957a) | |
| PAA80 | Alnus glutinosa | England | 1997 | J. Delcan (P950a) | |
| PAA81 | Alnus glutinosa | England | 1997 | J. Delcan (P937) | |
| PAA82 | Alnus glutinosa | England | 1996 | S. Gregory (P850) | |
| PAA85 | Alnus glutinosa | England | Unknown | C. Brasier (P834e) | |
| PAA91 | Alnus glutinosa | Hungary | 2001 | Z. Nagy (6d) | |
| PAA92 | A. glutinosa, soil | Hungary | 2001 | Z. Nagy (8d) | |
| PAA93 | A. glutinosa, soil | Hungary | 2001 | Z. Nagy (9d) | |
| PAA94 | A. glutinosa, soil | Hungary | 2001 | Z. Nagy (1ad) | |
| PAA95 | Alnus glutinosa | Hungary | 2001 | Z. Nagy (4-2d) | |
| PAA134 | Alnus glutinosa | Germany | 2000 | K. Kaminski (BBA 23/00) | |
| PAA162* | Alnus glutinosa | Germany | 2004 | R. Ioos (9a) | |
| PAA168 | Alnus glutinosa | Germany | 2004 | R. Ioos (8b) | |
| PAA141 | Alnus glutinosa | Austria | Unknown | T. Cech (Pucking B10) | |
| PAA143* | Alnus glutinosa | Poland | 2002 | G. Skuta (PO 192) | |
| PAA144 | Alnus glutinosa | Poland | 2003 | G. Skuta (PO 193) | |
| PAA145 | Alnus glutinosa | Poland | 2004 | G. Skuta (PO 203) | |
| PAA146 | Alnus glutinosa | Poland | 2002 | G. Skuta (PO 205) | |
| PAA189 | A. glutinosa, soil | Poland | 2004 | L. Orlikowski (P. alni soil) | |
| PAA190 | Alnus glutinosa | Poland | 2004 | L. Orlikowski (P. alni 5-yo) | |
| P. alni subsp. uniformis | PAU60* | Alnus glutinosa | France | 1999 | J. C. Streito (AUL028) |
| PAU84* | Alnus glutinosa | Sweden | 1997 | C. Olsson (P875a,b,c,f) | |
| PAU87 | Alnus glutinosa | Belgium | 2001 | D. De Merlier (2271c) | |
| PAU187 | Alnus glutinosa | Belgium | 2001 | D. De Merlier (2276c) | |
| PAU188 | Alnus incana | Belgium | 2001 | D. De Merlier (2277c) | |
| PAU89* | Alnus cordata | Italy | 2000 | P. Capretti (CBS109280e) | |
| PAU96 | Alnus glutinosa | Hungary | 1999 | Z. Nagy (155-ad) | |
| PAU97 | A. glutinosa, soil | Hungary | 1999 | Z. Nagy (155-bd) | |
| PAU98 | A. glutinosa, soil | Hungary | 1999 | Z. Nagy (155-cd) | |
| PAU142 | Alnus glutinosa | Slovenia | 2003 | A. Munda (Phy-A-Slo) | |
| P. alni subsp. multiformis | PAM54* | Alnus glutinosa | France | 2000 | J. C. Streito (DSFO/0125) |
| PAM71* | Alnus glutinosa, soil | The Netherlands | Unknown | W. Man in't Veld (W1139) | |
| PAM90 | Alnus glutinosa, soil | The Netherlands | Unknown | W. Man in't Veld (P972a,c,f) | |
| PAM73* | Alnus glutinosa | United Kingdom | 1996 | S. Gregory (P841a,c,f) | |
| PAM186 | Alnus glutinosa | Belgium | 2001 | D. De Merlier (2274c) | |
| P. cambivora | PC463 | Castanea sativa | France | 1994 | INRA Bordeaux |
| P. cambivora | PC643* | C. sativa, soil | France | 2000 | INRA Bordeaux |
| P. cambivora | PCjc17* | Quercus sp., soil | France | 1999 | C. Delatour |
| P. cambivora | PCGA1 | Quercus sp., soil | France | 1999 | C. Delatour |
| P. cambivora | PC99428 | Castanea sativa | France | 1999 | R. Ioos |
| P. cambivora | PCST3R1 | Quercus petraea | France | 1999 | C. Delatour |
| P. cambivora | PC627 | Castanea sativa | Italy | 2000 | INRA Bordeaux |
| P. cambivora | PC1A21 | Quercus sp., soil | France | 1999 | INRA Bordeaux |
| P. cambivora | PC4N1425 | Castanea sativa | France | 2004 | LNPV-UMAF |
| P. cambivora | PC4N444 | Castanea sativa | France | 2004 | LNPV-UMAF |
| P. fragariae var. fragariae | PFF1 | Fragaria × ananassa | United Kingdom | Unknown | K. Hughes |
| P. fragariae var. fragariae | PFF209.46 | Fragaria × ananassa | United Kingdom | 1946 | CBS (CBS209.46) |
| P. fragariae var. fragariae | PFF309* | Fragaria × ananassa | United Kingdom | 1962 | CBS (CBS 309.62) |
| P. fragariae var. rubi | PFRVR 59 | Rubus sp. | United Kingdom | Unknown | D. Cooke (FVR 59) |
| P. fragariae var. rubi | PFR163-2 | Rubus sp. | France | Unknown | A. Baudry (163-2) |
| P. fragariae var. rubi | PFR2 | Rubus sp. | United Kingdom | Unknown | K. Hughes |
| P. fragariae var. rubi | PFR967.95 | Rubus sp. | United Kingdom | 1985 | CBS (CBS967.95) |
| P. fragariae var. rubi | PFR109* | Rubus sp. | United Kingdom | 1991 | CBS (CBS109.892) |
| P. cactorum | CAC4810/TJ | Unknown | France | Unknown | C. Delatour |
| P. cinnamomi | DSFO2N0964 | Castanea sativa | France | 2002 | J. C. Streito |
| P. cinnamomi | DSFA970060 | Quercus suber | France | 1997 | J. C. Streito |
| P. cinnamomi | DSFO990050 | C. sativa, soil | France | 1999 | J. C. Streito |
| P. cinnamomi | P382 | Nothofagus procera, soil | United Kingdom | 1980 | C. Brasier |
| P. citricola | 2N0750-171 | Unknown | France | 2002 | J. C. Streito |
| P. citricola | AUL 045 AP7 | Alnus glutinosa | France | 1999 | J. C. Streito |
| P. citricola | 2AE5 | Quercus sp., soil | France | 1998 | C. Delatour |
| P. citricola | 3N1345-17 | Alnus glutinosa | France | 2003 | R. Ioos |
| P. citrophthora | 2N1021 | Rosa sp. | France | 2002 | J. C. Streito |
| P. cryptogea | 990675 | Actinidia chinensis | France | 1999 | J. C. Streito |
| P. erythroseptica | 960713 | Polygonum oberti | France | 1999 | J. C. Streito |
| P. europaea | AL5 | Quercus sp., soil | France | 1998 | C. Delatour |
| P. europaea | 2AU2 | Quercus sp., soil | France | 1999 | C. Delatour |
| P. gonapodyides | Gonap 4 | Quercus sp., soil | France | 1998 | C. Delatour |
| P. gonapodyides | AB4 | Quercus sp., soil | France | 1998 | C. Delatour |
| P. humicola | 3N1245-j | A. glutinosa, soil | France | 2003 | R. Ioos |
| P. ilicis | 3N1245-l | A. glutinosa, soil | France | 2003 | R. Ioos |
| P. inundata | 9500802 | A. glutinosa, soil | France | 1995 | J. C. Streito |
| P. lateralis | 98093.1-SPV | Chamaecyparis sp. | France | 1998 | J. C. Streito |
| P. megasperma | 3N1245-m | A. glutinosa, soil | France | 2003 | R. Ioos |
| P. megasperma | BK1 | Quercus sp., soil | France | 1998 | C. Delatour |
| P. megasperma | 03-12 | Water under Quercus sp. | France | 1998 | C. Delatour |
| P. megasperma | mega 1 | Unknown | Germany | 1998 | T. Jung |
| P. megasperma | 8RPOC3 | Quercus sp., soil | France | 1998 | C. Delatour |
| P. nicotianae | 960579 | Nicotiana tabacum | France | 1996 | J. C. Streito |
| Phytophthora taxon forestsoil | 8CARPPOC1 | Quercus sp., soil | France | 1998 | C. Delatour |
| P. palmivora | 970423 | Hedera sp. | France | 1997 | J. C. Streito |
| P. parasitica | 970029 | Lycopersicon esculentum | France | 1997 | J. C. Streito |
| Phytophthora taxon Pgchlamydo | Haye,3,1 | Quercus sp., soil | France | 1998 | C. Delatour |
| P. pseudosyringae | EW5 | Quercus sp., soil | France | 1998 | C. Delatour |
| P. psychrophila | FF20 | Quercus sp., soil | France | 1998 | C. Delatour |
| P. quercina | FNA | Quercus sp., soil | France | 1999 | C. Delatour |
| P. quercina | Mers2 | Quercus sp., soil | France | 1999 | C. Delatour |
| P. ramorum | 2N0983 | Rhododendron sp. | France | 2002 | C. Saurat |
| P. ramorum | 3N0003 | Viburnum sp. | France | 2002 | C. Saurat |
| P. sojae | 443 | Glycine max | Unknown | Unknown | F. Panabières |
| P. syringae | 2JZ2 | Quercus sp., soil | France | 1999 | C. Delatour |
| Pythium aphanidermatum | Ctsa | Unknown | France | 2003 | S. Verger |
| Pythium sylvaticum | 0675/a | Unknown | France | 2003 | S. Verger |
| Pythium intermedium | 02/84/1 | Unknown | France | Unknown | S. Verger |
| Pythium irregulare | 02/57/1 | Unknown | France | Unknown | S. Verger |
| Pythium ultimum | 433/3 | Unknown | France | Unknown | S. Verger |
| Pythium sp. | 3N1345-11 | A. glutinosa, soil | France | 2003 | R. Ioos |
Also studied by Delcan and Brasier (9).
Also studied by Brasier et al. (3).
Also studied by De Merlier et al. (10).
Also studied by Nagy et al. (22).
Also studied by Santini et al. (30).
Also studied by Brasier and Kirk (4).
*, isolate used for mRNA production in this study and also studied by Ioos et al. (15).
Nucleic acid manipulation.
Genomic DNA was extracted from 5-day-old cultures grown in shake culture in liquid V8 juice medium (21) at 20°C using a plant DNA extraction kit (DNeasy plant minikit; QIAGEN, Courtaboeuf, France) by following the manufacturer's instructions with slight modifications. Briefly, ca. 200 mg of fresh mycelium was harvested and mixed in a 2-ml tube with 400 μl of lysis buffer and 4 μl of the RNase A provided with the kit. The mixture was ground for 2 min with two 3-mm tungsten carbide beads at a frequency of 30 Hz, using a mixer mill grinder (Tissuelyser; QIAGEN). The ground solution was subsequently centrifuged for 5 min at 15,000 × g to compact the debris, and the resulting supernatant was treated in accordance with the manufacturer's instructions. Genomic DNA was stored at −20°C until used for PCR tests.
To enhance elicitin mRNA synthesis, oomycete cultures were grown for 3 days in shake culture in liquid elicitin secretion medium (ESM; M. Horta [Algarve University, Portugal], personal communication) at 20°C. The composition of the ESM was 0.05% (wt/vol) KH2PO4, 0.025% (wt/vol) MgSO4·7H2O, 0.1% (wt/vol) asparagine, 1 mg/liter thiamine, 0.05% (wt/vol) yeast extract, and 2% (wt/vol) glucose. The medium was sterilized by filtration through a 0.2-μm membrane. mRNAs were extracted using a QuickPrep micro-mRNA purification kit with oligo(dT) cellulose (Amersham Biosciences, Orsay, France) in accordance with the manufacturer's instructions, resuspended in 40 μl of diethyl pyrocarbonate-treated molecular biology grade water, and stored at −80°C.
Reverse transcription-PCR, cloning, and sequencing of elicitin mRNA.
Polyadenylated RNAs were reverse-transcribed using a SuperScript first-strand synthesis system (Invitrogen, Cergy Pontoise, France) with NotI-oligo(dT) [5′-ATTCGCGGCCGCAGGA(T)16-3′] (25). A PCR was performed on the cDNA template using a combination of the NotI-oligo(dT) primer and degenerate primer 1 (5′-ATGAACTTCCGCGCTCTSYTYGC-3′), initially designed from conserved sequences of class I elicitins located in the peptide signal region (25). This primer was assumed to efficiently anneal to the peptide signal region of every elicitin class I gene unraveled until now. PCRs were carried out in a 20-μl PCR mixture containing 1× polymerase buffer (Sigma-Aldrich, L'Isle d'Abeau, France), 0.9 mM MgCl2, 0.3 μM of each primer, 180 μM deoxynucleoside triphosphates, 0.6 unit of Taq DNA polymerase (Sigma-Aldrich), and 2 μl of template cDNA. Molecular biology grade water was added to 20 μl. For each isolate, PCRs were also performed using genomic DNA as a negative control to test potential amplification of genomic DNA in cDNA amplification. The PCRs were carried out with a GeneAmp 9700 thermal cycler (Applied Biosystems, Foster City, CA). The cycling profile included an initial denaturation step at 95°C for 2 min, followed by 35 cycles of denaturation, annealing, and elongation for, respectively, 20 s at 94°C, 30 s at 60°C, and 1 min at 72°C and a final extension step at 72°C for 7 min. PCR fragments were separated by a 1-h electrophoresis on a 1% agarose gel at 4 V·cm−1. Gels were stained with ethidium bromide, and images were recorded with a charge-coupled device camera and a GELDOC 2000 system (Bio-Rad, Marne-La-Coquette, France).
The PCR products generated with cDNAs were cloned for each of the 15 isolates tested using the pCR4-TOPO TA cloning kit (Invitrogen). Five microliters of the PCR product was transferred into a sterile 1.5-ml microcentrifuge tube, and the amplicons were ligated to a TOPO vector (Invitrogen) and used to transform TOP 10-competent cells (Invitrogen) according to the manufacturer's instructions. Positive clones were selected by PCR amplification of inserts with M13 sequencing primers. Positive clones were selected according to their expected PCR product sizes, corresponding to class I elicitin transcripts. PCR products were purified by centrifugation using a polyethylene glycol 8000 solution as described by Rosenthal et al. (29). Double-stranded DNA sequencing was performed by the dideoxy chain termination method using a T3-T7 sequencing kit on a CEQ 2000 XL DNA sequencer (Beckman, Fullerton, CA). Sequences were edited with Sequencher software (Gene Codes, Ann Arbor, MI) and aligned using ClustalW (33) (Table 2). The cDNA sequences were translated using Fast PCR software, version 3.6.62 (R. Kalendar, FastPCR, PCR primer design, DNA and protein tools, repeats and own database search program [www.biocenter.helsinki.fi/bi/programs/fastpcr.htm]). The isoelectric points (pI) of the deduced proteins were calculated using IEP online software (http://bioweb.pasteur.fr/seqanal/interfaces/iep.html). Multiple amino acid sequence alignments with hierarchical clustering were performed using MultAlin program, version 5.3.3 (8), with Blossum 62 as symbol comparison table. An unrooted phylogram was built using a parsimony analysis and the neighbor-joining method in PAUP* 4.0b10 (32). Bootstrap values were computed on 10,000 replicates.
TABLE 2.
Characteristics of all the different cDNA and genomic sequences obtained and assignation to a specific 3′UTR group
| Taxon | Isolate | Elicitin | 3′UTR group | GenBank accession no. of:
|
|
|---|---|---|---|---|---|
| cDNA sequence | Genomic sequence | ||||
| P. alni subsp. alni | PAA129 | AE1.1 | a2 | DQ012518 | |
| AE1.1 | a10′ | EF158402 | |||
| AE1.2 | a2 | EF158401 | |||
| AE2 | a3 | DQ012517 | |||
| PAA130 | AE1.1 | a1 | EF158403 | ||
| AE1.1 | a10′ | EF158404 | |||
| AE1.2 | a1 | DQ012520 | |||
| AE1.2 | a5 | DQ012519 | |||
| BE1 | b2 | EF158405 | |||
| PAA143 | AE1.2 | a1 | DQ012521 | ||
| AE1.2 | a2 | DQ012522 | |||
| AE2 | a4 | EF158406 | |||
| PAA151 | AE1.2 | a2 | DQ012524 | ||
| AE1.2 | a3′ | DQ012525 | |||
| BE2 | b1 | DQ012523 | |||
| PAA162 | AE1.2 | a2 | DQ012527 | ||
| BE1 | b1 | DQ012526 | |||
| P. alni subsp. multiformis | PAM54 | AE1.1 | a1 | DQ012508 | |
| AE1.1 | a2 | EF158407 | |||
| AE1.2 | a2 | EF158408 | |||
| AE1.2 | a3′ | EF158409 | |||
| AE2 | a3 | EF158410 | |||
| BE1 | b1 | EF158411 | |||
| HAE1 | ha1 | DQ012509 | |||
| PAM71 | AE1.1 | a8 | EF158415 | ||
| AE1.2 | a3′ | EF158413 | |||
| AE2 | a4 | DQ012510 | |||
| AE2 | a3 | EF158414 | |||
| BE1 | b1 | DQ012511 | |||
| BE1 | b1 | EF158412 | |||
| PAM73 | AE2 | a3 | DQ012513 | ||
| BE1 | b1 | DQ012512 | |||
| P. alni subsp. uniformis | PAU60 | AE1.1 | a1 | EF158417 | |
| BE1 | b2 | EF158416 | |||
| PAU84 | AE1.1 | a1 | DQ012514 | ||
| AE1.1 | a1 | EF158418 | |||
| AE1.1 | a10′ | EF158419 | |||
| PAU89 | AE1.1 | a1 | DQ012516 | ||
| AE1.1 | a1 | EF158420 | |||
| HAE1 | ha1 | DQ012515 | |||
| PAU142 | AE1.1 | a1 | EF158421 | ||
| PAU188 | AE1.1 | a1 | EF158422 | ||
| P. cambivora | PC643 | AE1.1 | a6 | DQ012529 | |
| AE1.1 | a8 | DQ012528 | |||
| AE1.1 | a2′ | EF158424 | |||
| AE2 | a10″ | EF158423 | |||
| PCjc17 | AE1.1 | a6 | DQ012530 | ||
| AE1.1 | a7 | DQ012531 | |||
| AE1.1 | a8 | EF158425 | |||
| P. fragariae var. fragariae | PFF309 | AE2 | a9 | DQ012532 | |
| AE2 | a11 | DQ012533 | |||
| P. fragariae var. rubi | PFR109 | AE1.1 | a9 | DQ012535 | |
| AE1.1 | a10 | EF158426 | |||
| AE1.1 | a10 | DQ012534 | |||
3′UTR-specific PCR detection and sequencing.
Based on cDNA sequence alignment, a set of 11 reverse primers was designed to target 3′UTR-specific regions among elicitin-encoding sequences (Table 3). Primers were synthesized by Invitrogen and tested on the 15-isolate panel, then on the entire Phytophthora and Pythium collection listed in Table 1.
TABLE 3.
List and sequences of the 3′UTR-specific reverse primers designed in this study
| Elicitin class | Code | Sequence (5′-3′) |
|---|---|---|
| I, acidic | a2-R | AGG GTG GAT GGG GGA TTG CCA |
| a3-R | CGA AGA CAC GTC GGT ATC CAT | |
| a4-R | GAC AAG TCG GCA TAA CAA AC | |
| a5-R | GCT CAG ACA ACA CTC AAG CT | |
| a7-R | GCT GAA ACA ATG CTC AAG A | |
| a8-R | GCT GAT CTG AAG ACG AGT C | |
| a10-R | GCT GCG TAC TTA GTC CAC GC | |
| a11-R | CTG CAT CGG AAT TCC AAC AAC | |
| I, basic | b1-R | CTT CGA GTT AAT GGC GTA TTA |
| b2-R | CCT TGA GTT TTA ATG GTA GA | |
| II, highly acidic | ha1-R | GTG ACG TCG CGC CTG ATC CAG |
The 3′UTR PCR tests were carried out in a 20-μl mixture containing 1× polymerase buffer (Sigma-Aldrich), 1.8 mM MgCl2, 0.45 μM of degenerate primer 1, 0.45 μM of 3′UTR-specific primer, 180 μM deoxynucleoside triphosphates, 0.7 μg·μl−1 bovine serum albumin (Sigma-Aldrich), 0.6 unit of Taq DNA polymerase (Sigma-Aldrich), and 2 μl of template genomic DNA or cDNA. Molecular biology grade water was added to 20 μl. PCR parameters were as indicated above except that the annealing temperature was lowered to 58°C. PCR products were resolved by agarose gel electrophoresis as described above.
In order to verify the specificity of the 3′UTR-specific PCR assays, some of the PCR products were subsequently sequenced using degenerate primer 1 as the sequencing primer.
Nucleotide sequence accession numbers.
mRNA sequences generated in this study were deposited in GenBank under accession numbers DQ012508 to DQ012535. PCR products sequenced with degenerate primer 1 as the sequencing primer were deposited in GenBank under accession numbers EF158401 to EF158426.
RESULTS
Cloning, sequencing, and classification of elicitin-encoding sequences.
A subset of 15 Phytophthora isolates (Table 1) was selected to study the elicitin expression pattern through mRNA sequencing. Using a combination of oligo(dT) primer and a degenerate oligonucleotide designed ahead of the 5′ end of the elicitin coding sequence, reverse transcription-PCR of the mRNA extracted for each of these strains showed a strong smeared signal corresponding to a 450- to 550-bp product. For each isolate, the entire amplicon was cloned and 32 individual clones were randomly selected and checked for insert size. Inserts ranged from ca. 480 to 570 bp. Therefore, for each isolate, one to three clones of different sizes were selected for sequencing. A total of 28 different sequences were obtained with clones generated from the 15-isolate panel that were translated along with two unpublished class I elicitin sequences from P. cambivora isolate 143 (PC_cam1) and P. fragariae var. rubi isolate 486 (PFR_fra1) (F. Panabières, unpublished data).
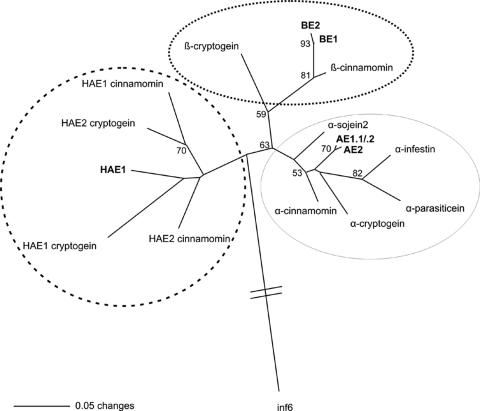
Translation of the 28 cDNA sequences resulted in five distinct amino acid sequences (Fig. 1), which belonged to the class I (98 amino acids [aa]) and class II (99 aa) elicitins, according to the classifications of Ponchet et al. (26) and Qutob et al. (27). The unrooted phylogram based on parsimony analysis separated the sequences into three classes, corresponding to acidic, basic, and highly acidic elicitins (Fig. 2).
FIG. 1.
Multiple-amino-acid sequence alignment of the elicitins characterized in this study for P. alni, P. cambivora, and P. fragariae (AE1.1, AE1.2, AE2, BE1, BE2, and HAE1) and well documented acidic (α), basic (β), and highly acidic (HAE) elicitins retrieved from the GenBank database: P. cryptogea Z34462, Z34459, Z34460, Z34461 (24); P. infestans AY830090 (17); P. cinnamomi AJ000071 (11); P. sojae AJ007859 (1); and P. parasitica S67432 (6). The sequence corresponding to the signal peptide is underlined. pI are indicated in parentheses, following sequence references.
FIG. 2.
Phylogenetic relationships between elicitins, inferred from the sequence alignment of the mature polypeptides. The unrooted phylogram was constructed using a parsimony analysis and the neighbor-joining method, based on the multiple alignment of elicitin sequences listed in Fig. 1 and using the P. infestans inf6 sequence as an outgroup. Bootstrap values (>50%) from 10,000 replicates are indicated. AE1.1/0.2 and AE2 fall into the class I acidic elicitin group (solid line), whereas BE1 and BE2 are class I basic elicitins (dotted line). HAE1 is closely related to class II highly acidic elicitins (dashed line).
First, two amino acid sequences deduced from five cDNA clones, including PC_cam1, were identified as basic elicitins. They displayed a predicted pI of 8.22 and several key signatures such as the K13 frequently observed in basic elicitins (26). The two proteins differed only by a single A84V substitution and were therefore designated BE1 and BE2 (basic elicitin).
An alignment of the 98-aa core region corresponding to the mature protein (excluding the 20-aa peptide signal sequence) allowed the identification of two other proteins, differing by a single S40A substitution, among a set of 23 sequences. From alignment with published sequences and from their predicted pI of 4.99, they were clearly identified as acidic elicitins and named AE1 and AE2 (acidic elicitin). Extending the comparison to the entire amino acid sequence further split AE1 into two proteins, which differed by a single S17F mutation in the peptide signal sequence, and these were designated AE1.1 and AE1.2.
Last, a third type of elicitin protein was identified. The deduced peptide comprised 119 aa, including the peptide signal sequence, and displayed a predicted pI of 3.95. From alignment with known elicitin sequences, it was considered to belong to class II of highly acidic elicitins and was accordingly called HAE1 (11, 26).
Elicitin-encoding mRNAs display important 3′UTR variability.
The diversity of elicitin-encoding sequences was also investigated at the nucleotide level on the 3′UTR (Fig. 3).
FIG. 3.
Sequence alignment of the regions corresponding to the 3′UTRs of the mRNAs, deduced from cDNA sequencing. 3′UTR-specific groups are indicated on the left and correspond to the clustering of identical or nearly identical sequences. Fourteen different 3′UTR groups were defined among the 28 sequences obtained from our 15-isolate panel. The different 3′UTR groups were designated according to the elicitin class, i.e., a for acidic, ha for highly acidic, and b for basic elicitins. For each 3′UTR, the boxed sequence represents the polymorphic region from which a 3′UTR-specific primer could be designed. The PFF309b 3′UTR sequence was so divergent that it could not be properly aligned. PFF309b was therefore not represented, but a specific reverse primer could be designed (a11-R).
First, the two cDNA sequences encoding the putative highly acidic elicitin, including the 3′UTR, were identical. This 3′UTR region was consequently designated ha1.
Second, sequences encoding basic elicitins were highly similar, diverging by one to four synonymous substitutions and by a C/T transversion leading to the A84V substitution in the coding region. In addition, the 3′UTRs of the different clones derived from P. alni sensu lato were strictly identical over the entire 168-bp region, with the exception of a single G/C transversion in the transcript obtained from isolate PAA162 (data not shown). This 3′UTR sequence was designated b1. The PC_cam1 sequence was more divergent, displaying 8 synonymous substitutions in the coding region and 14 substitutions, as well as a 5-bp deletion, in the 3′UTR. This 3′UTR sequence was called b2.
Last, for acidic elicitins, 11 clones encoding AE1.1 and 7 cDNA clones encoding AE1.2 were examined. They displayed extensive conservation in the coding region, as only three synonymous mutations further differentiated the two groups. The polymorphism was much higher in the 3′UTRs, which could be classified into 11 3′UTR groups, called a1 to a11. The diversity was the outcome of frequent insertions and deletions as well as single-nucleotide polymorphisms.
Overall, there was no obvious correlation between the classification of 3′UTRs and the protein sequence deduced from the coding region (Table 2). AE2 was associated with a3, a4, a9, and a11 3′UTRs. Similarly, AE1.1 was associated with a1, a2, a6, a7, a8, a9, and a10 3′UTRs, while AE1.2 was associated with a1, a2, a3′, and a5 3′UTRs. As a consequence, a given 3′UTR could be associated with two different protein sequences.
Expression and distribution of the different elicitin genes.
The occurrence of the whole set of genes revealed by sequencing of cDNAs was further investigated through 3′UTR-specific PCR tests. Specific primers could be designed for 11 out of the 14 3′UTR groups (Table 3). PCR assays were carried out first with the cDNAs from the 15 isolates of the panel, then with genomic DNA extracted from a large set of 101 P. alni subsp. alni, P. alni subsp. multiformis, P. alni subsp. uniformis, P. cambivora, P. fragariae var. fragariae, and P. fragariae var. rubi isolates and other Phytophthora and Pythium species (Table 1).
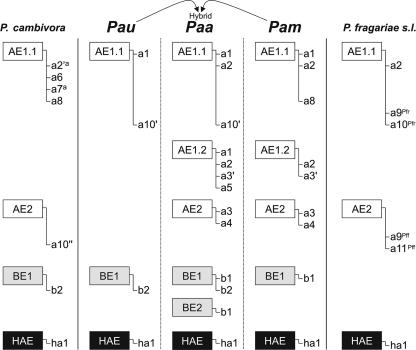
Results of the 3′UTR-specific PCR tests with cDNAs for P. alni subsp. alni, P. alni subsp. multiformis, and P. alni subsp. uniformis were identical for all isolates of a given taxon, suggesting that the diversity observed in the elicitin-encoding sequences does not correspond to individual variation, but rather to a high level of elicitin complexity among the three taxa (Fig. 4).
FIG. 4.
Distribution of the acidic, basic, and highly acidic elicitin genes resolved in this study for P. alni subsp. alni (Paa), P. alni subsp. multiformis (Pam), P. alni subsp. uniformis (Pau), and the phylogenetically close species P. cambivora and P. fragariae sensu lato, as inferred from sequencing of cDNA and 3′UTR-specific PCR tests conducted on genomic DNA and cDNA libraries. Coding sequences are boxed and were found to be associated with different 3′UTRs. The occurrence of a1, a6, and a9 3′UTRs is deduced only from sequencing, as no 3′UTR-specific primer could be designed from these sequences. With the exception of the HAE1 associated with the ha1 3′UTR in P. cambivora, all the elicitin genes we resolved were shown to be expressed in our 15-isolate panel by 3′UTR-specific PCR with cDNA libraries. a, only for P. cambivora isolate PCjc17; Pff, obtained for P. fragariae var. fragariae; Pfr, obtained for P. fragariae var. rubi.
In addition, the results of 3′UTR-specific PCR tests conducted on genomic DNA of the 101 Phytophthora and Pythium isolates (Table 1) were in complete agreement with those obtained on the cDNAs from the 15-isolate panel and showed that, except for P. cambivora, the elicitin gene patterns were conserved among the different isolates of each taxon (Fig. 4). Sequencing of a subset of 3′UTR-specific PCR products generated with genomic DNA extracts confirmed their assignation to specific 3′UTRs and resolved three additional 3′UTR sequences, called a2′, a3′, and a10′ and a10′′, slightly different from the original cDNA sequences a2, a3, and a10, respectively (Table 2).
The distributions of the various acidic elicitin-related 3′UTRs with cDNA and with genomic DNA were identical, demonstrating that the whole elicitin gene content was actually expressed in the current culture conditions. As a single exception, ha1 was amplified from the genomic DNA of P. cambivora isolates, whereas it was not detected in the cDNA, indicating that, for this species, this class of genes was not expressed during our vegetative-growth conditions.
Overall, P. alni subsp. alni displayed an elicitin gene pattern that combined those observed for P. alni subsp. multiformis and for P. alni subsp. uniformis, with the exception of the a8 3′UTR, which could not be observed in P. alni subsp. alni, due to unexpected cross-annealing of the a8-R PCR primer with the a1 3′UTR sequence, and of the a5 3′UTR, which could not be observed in P. alni subsp. multiformis, due to unexpected cross-annealing with the a3′ 3′UTR sequence. These cross-annealing events were suggested by in silico annealing tests and further unambiguously demonstrated by sequencing the PCR products (data not shown). Additionally, the occurrence of the BE2 elicitin gene in P. alni subsp. multiformis or in P. alni subsp. uniformis could not be verified since all the sequenced products generated by b1-specific PCR corresponded to a gene encoding BE1 (Table 2). The BE2-encoding sequence may be either (i) present in the hybrid while not present in the progenitors, thus representing an autapomorphic feature (a derived characteristic unique to a given taxon or monophyletic group) that would have been generated during or after the hybridization event, or (ii) not detectable by sequencing PCR products because of underrepresentation in comparison with BE1-encoding sequences.
In contrast with P. alni sensu lato, analysis of P. cambivora and P. fragariae cDNAs revealed a patchy distribution of the various acidic elicitin-related 3′UTRs. The a8 3′UTR was detected in the cDNAs from the two strains of P. cambivora (the sequence of a6 previously obtained from these strains did not allow the design of specific primers), but only isolate PCjc17 appeared to express additional 3′UTRs, namely, a2′ and a7 3′UTRs. Similarly P. fragariae var. fragariae and P. fragariae var. rubi isolates shared a2 and a9 3′UTRs (whose sequences did not allow the design of specific primers), but only the P. fragariae var. rubi isolate expressed an additional gene containing a10, while a11 appeared to be specific to the P. fragariae var. fragariae isolate.
Finally, 3′UTR-specific PCRs did not yield any positive signal with other Phytophthora or Pythium species (Table 1), confirming that the primers designed in this study are specific to P. alni subsp. alni, P. alni subsp. multiformis, P. alni subsp. uniformis, P. cambivora, and P. fragariae and that these five taxa share particular evolutionary relationships.
DISCUSSION
Taken as a whole and inferred both from sequence data and from 3′UTR-specific PCR amplifications, the elicitin gene family is more diverse in P. alni subsp. alni and P. alni subsp. multiformis than in the diploid or nearly diploid taxa P. alni subsp. uniformis, P. cambivora, P. fragariae var. fragariae, and P. fragariae var. rubi. This complexity is consistent with the hybrid status for P. alni subsp. alni but also supports the hypothesis that P. alni subsp. multiformis is probably also an ancient allopolyploid taxon (15). In addition, most of the mRNA patterns observed in the allopolyploid hybrid P. alni subsp. alni are a composite of the mRNA patterns from its putative progenitors, P. alni subsp. multiformis and P. alni subsp. uniformis. In this respect, we showed that, at least for the elicitin genes, the different genomes are currently expressed in the hybrid P. alni subsp. alni. However, additional data would be needed to demonstrate that the entire distinct genome sets are still coexpressed in P. alni subsp. alni. Garcia-Olmedo et al. (12) and Volkov et al. (34) showed that, in the course of evolution of protein-coding genes in allopolyploid plants, the alleles of one parental species are mainly transcriptionally active, whereas the alleles from the other parent are gradually transformed into pseudogenes. By contrast, the results presented here suggest that gene silencing is not yet observed for this highly expressed family of elicitin genes in the allopolyploid hybrid P. alni subsp. alni. These results strengthen the hypothesis that this taxon is of recent origin and still evolving (3), which is in good accordance with its recent emergence as an aggressive alder pathogen (14).
More unexpected, from an evolutionary point of view, is the association of given 3′UTRs, namely, those of a1 and a2, with two distinct coding regions in the hybrid P. alni subsp. alni and in P. alni subsp. multiformis, since a higher selection pressure is assumed to have been exerted on the coding regions. In this respect, it may be hypothesized that recent (P. alni subsp. alni) or likely more ancient (P. alni subsp. multiformis) hybridization events may have been followed by recombination with coding sequences from paralogs present in the putative parental species. In polyploid plants and animals, chromosomal reorganization and gene silencing generally occur rapidly and may be so extensive that the genome is no longer structured as an allopolyploid (31). P. alni subsp. alni still appears structured as an allopolyploid taxon since previous studies demonstrated that P. alni subsp. alni combined the alleles of its progenitors, P. alni subsp. uniformis and P. alni subsp. multiformis, for a series of single-copy genes (15) and for microsatellite loci (16). However, this study demonstrates that, similar to the additivity observed in the ribosomal DNA internal transcribed spacer (3), at least for the allopolyploid taxon P. alni subsp. alni, genetic recombination also occurred between paralogs of the elicitin genes.
Except for the observed new combinations between coding sequences and 3′UTRs, the genomic elicitin pattern of P. alni subsp. alni combined as expected those of the putative parental taxa, P. alni subsp. multiformis and P. alni subsp. uniformis. In addition, while P. alni subsp. uniformis shared several 3′UTRs with P. cambivora, P. alni subsp. alni and P. alni subsp. multiformis possessed private sequences, e.g., a3, a4, and b1. These sequences were found neither in P. cambivora nor in P. fragariae. These findings confirm the close relationship between P. cambivora and P. alni subsp. uniformis (3, 15). Conversely, both P. cambivora and P. fragariae displayed specific elicitin sequences not found in P. alni subsp. alni, in agreement with previous results rejecting the hypothesis that these species may have been the putative parents of the hybrid P. alni subsp. alni (15).
Furthermore, P. alni subsp. alni, P. alni subsp. multiformis, P. alni subsp. uniformis, P. cambivora, and P. fragariae seem to have retained identical genes independently of the speciation. This phenomenon has been partially shown for P. cactorum and P. pseudotsugae (26), but cactorein and pseudotsugaein genes possess distinct 3′UTR sequences (F. Panabières, unpublished data). Jiang et al. (18) demonstrated that the main diversification events of elicitin genes occurred before Phytophthora radiation and that elicitin genes of a given clade, such as the one analyzed in the present work, are under purifying selection. In this respect, the duplication of elicitin genes, creating paralogs, could also explain the multiplicity of divergent 3′UTR sequences for a given elicitin gene, with the assumption of a lower selection pressure on this part of the gene. It is likely that the diversity of elicitin genes in P. cambivora, P. fragariae, and P. alni reflects duplication events prior to the radiation of these species from their common ancestor. It would also indicate that the radiation of these species is of particularly recent origin (7), which seems to be supported by the cross-amplification of microsatellite markers in all three species (16). However, the unexpected conservation of several 3′UTR regions for these different species (e.g., ha1, b2, a8) could also be the outcome of reticulation or introgression events. Gene transfer after speciation should not be ruled out as a possibility to explain the elicitin gene patterns observed within this clade.
Acknowledgments
This work was supported by grants from INRA, the Agence de l'Eau Rhin-Meuse, and the Institut Fédérateur de Recherches 110.
We thank the colleagues listed in Table 1 for sharing Phytophthora sp. isolates. We are grateful to Everett Hansen for his critical reading of an early version of the manuscript and to Christine Delaruelle for her help in sequencing.
Footnotes
Published ahead of print on 29 June 2007.
REFERENCES
- 1.Becker, J., S. Nagel, and R. Tenhaken. 2000. Cloning, expression and characterization of protein elicitors from the soybean pathogenic fungus Phytophthora sojae. J. Phytopathol. 148:161-167. [Google Scholar]
- 2.Brasier, C. M. 2003. The hybrid alder Phytophthora: genetic status, pathogenicity, distribution, and competitive survival, p. 39-54. In J. Gibbs, C. Van Dijk, and J. Webber (ed.), Phytophthora disease of alder in Europe. Bulletin 126. Forestry Commission, Edinburgh, United Kingdom.
- 3.Brasier, C. M., D. E. L. Cooke, and J. M. Duncan. 1999. Origin of a new Phytophthora pathogen through interspecific hybridization. Proc. Natl. Acad. Sci. USA 96:5878-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brasier, C. M., and S. Kirk. 2001. Comparative aggressiveness of standard and variant hybrid alder Phytophthora, Phytophthora cambivora and other Phytophthora species on bark of Alnus, Quercus and other woody hosts. Plant Pathol. 50:218-229. [Google Scholar]
- 5.Brasier, C. M., S. A. Kirk, J. Delcan, D. E. L. Cooke, T. Jung, and W. A. Man in't Veld. 2004. Phytophthora alni sp. nov. and its variants: designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycol. Res. 108:1172-1184. [DOI] [PubMed] [Google Scholar]
- 6.Colas, V., S. Conrod, P. Venard, H. Keller, P. Ricci, and F. Panabières. 2001. Elicitin genes expressed in vitro by certain tobacco isolates of Phytophthora parasitica are down regulated during compatible interactions. Mol. Plant-Microbe Interact. 14:326-335. [DOI] [PubMed] [Google Scholar]
- 7.Cooke, D. E. L., A. Drenth, J. M. Duncan, G. Wagels, and C. M. Brasier. 2000. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Biol. 30:17-32. [DOI] [PubMed] [Google Scholar]
- 8.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delcán, J., and C. M. Brasier. 2001. Oospore viability and variation in zoospore and hyphal tip derivatives of the hybrid alder phytophthoras. For. Pathol. 31:65-83. [Google Scholar]
- 10.De Merlier, D., A. Chandelier, N. DeBruxelles, L. Noldus, F. Laurent, E. Dufays, H. Classens, and M. Cavelier. 2005. Characterization of alder Phytophthora isolates from Wallonia and development of SCAR primers for their specific detection. J. Phytopathol. 153:99-107. [Google Scholar]
- 11.Duclos, J., A. Fauconnier, A. C. Coelho, A. Bollen, A. Cravador, and E. Godfroit. 1998. Identification of a gene cluster in Phytophthora cinnamomi. DNA Seq. 9:231-237. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Olmedo, F., P. Carbonero, C. Aragancillo, and G. Salcedo. 1978. Loss of redundant gene expression after polyploidization in plants. Experentia 34:332-333. [Google Scholar]
- 13.Gibbs, J., C. Van Dijk, and J. Webber. 2003. Phytophthora disease of alder in Europe. Bulletin 126. Forestry Commission, Edinburgh, United Kingdom.
- 14.Gibbs, J. N. 1995. Phytophthora root disease of alder in Britain. EPPO Bull. 25:661-664. [Google Scholar]
- 15.Ioos, R., A. Andrieux, B. Marçais, and P. Frey. 2006. Genetic characterization of the natural hybrid species Phytophthora alni as inferred from nuclear and mitochondrial DNA analyses. Fungal Genet. Biol. 43:511-529. [DOI] [PubMed] [Google Scholar]
- 16.Ioos, R., B. Barrès, A. Andrieux, and P. Frey. 2007. Characterization of microsatellite markers in the hybrid Phytophthora alni subsp. alni and cross-amplification with related taxa. Mol. Ecol. Notes 7:133-137. [Google Scholar]
- 17.Jiang, R. H., A. L. Dawe, R. Weide, M. van Staveren, S. Peters, D. L. Nuss, and F. Govers. 2005. Elicitin genes in Phytophthora infestans are clustered and interspersed with various transposon-like elements. Mol. Genet. Genomics 273:20-32. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, R. H. Y., B. M. Tyler, S. C. Whisson, A. R. Hardham, and F. Govers. 2006. Ancient origin of elicitin gene clusters in Phytophthora genomes. Mol. Biol. Evol. 23:338-351. [DOI] [PubMed] [Google Scholar]
- 19.Man in't Veld, W. A., A. W. A. M. de Cock, and R. C. Summerbell. 2007. Natural hybrids of resident and introduced Phytophthora species proliferating on new hosts. Eur. J. Plant Pathol. 117:25-33. [Google Scholar]
- 20.Man in't Veld, W. A., W. J. Veenbaas-Rijk, E. Ilieva, A. W. A. M. De Cock, P. J. M. Bonants, and R. Pieters. 1998. Natural hybrids of Phytophthora nicotianae and P. cactorum demonstrated by isozyme analysis and random amplified polymorphic DNA. Phytopathology 88:922-929. [DOI] [PubMed] [Google Scholar]
- 21.Miller, P. M. 1955. V-8 juice agar as a general purpose medium for fungi and bacteria. Phytopathology 45:461-462. [Google Scholar]
- 22.Nagy, Z. A., J. Bakonyi, and T. Ersek. 2003. Standard and Swedish variant types of the hybrid alder Phytophthora attacking alder in Hungary. Pest Manag. Sci. 59:484-492. [DOI] [PubMed] [Google Scholar]
- 23.Panabières, F., J. Amselem, E. Galiana, and J. Y. Le Berre. 2005. Gene identification in the oomycete pathogen Phytophthora parasitica during in vitro vegetative growth through expressed sequence tags. Fungal Genet. Biol. 42:611-623. [DOI] [PubMed] [Google Scholar]
- 24.Panabières, F., A. Marais, J. Y. Le Berre, I. Penot, D. Fournier, and P. Ricci. 1995. Characterization of a gene cluster of Phytophthora cryptogea which codes for elicitins, proteins inducing a hypersensitive-like response in tobacco. Mol. Plant-Microbe Interact. 8:996-1003. [DOI] [PubMed] [Google Scholar]
- 25.Panabières, F., M. Ponchet, V. Allasia, L. Cardin, and P. Ricci. 1997. Characterization of border species among Pythiaceae: several Pythium isolates produce elicitins, typical proteins from Phytophthora spp. Mycol. Res. 101:1459-1468. [Google Scholar]
- 26.Ponchet, M., F. Panabières, M. L. Milat, J. L. Montillet, L. Suty, C. Triantaphylides, Y. Tirilly, and J. P. Blein. 1999. Are elicitins cryptogams in plant-oomycete communications? Cell. Mol. Life Sci. 56:1020-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qutob, D., E. Huitema, M. Gijzen, and S. Kamoun. 2003. Variation in structure and activity among elicitins from Phytophthora sojae. Mol. Plant Pathol. 4:119-124. [DOI] [PubMed] [Google Scholar]
- 28.Robin, C., M. L. Desprez-Loustau, G. Capron, and C. Delatour. 1998. First record of Phytophthora cinnamomi on cork and holm oak in France and evidence of pathogenicity. Ann. Sci. For. 55:869-883. [Google Scholar]
- 29.Rosenthal, A., O. Coutelle, and M. Craxton. 1993. Large-scale production of DNA sequencing templates by microtitre format PCR. Nucleic Acids Res. 21:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santini, A., G. P. Barzanti, and P. Capretti. 2003. Susceptibility of some mesophilic hardwoods to alder Phytophthora. J. Phytopathol. 151:406-410. [Google Scholar]
- 31.Soltis, D. E., and P. S. Soltis. 1999. Polyploidy: recurrent formation and genome evolution. Trends Ecol. Evol. 9:348-352. [DOI] [PubMed] [Google Scholar]
- 32.Swofford, D. L. 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer Associates, Sunderland, MA.
- 33.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkov, R. A., N. V. Borisjuk, I. I. Panchuk, D. Schweizer, and V. Hemleben. 1999. Elimination and rearrangement of parental rDNA in the allotetraploid Nicotiana tabacum. Mol. Biol. Evol. 16:311-320. [DOI] [PubMed] [Google Scholar]