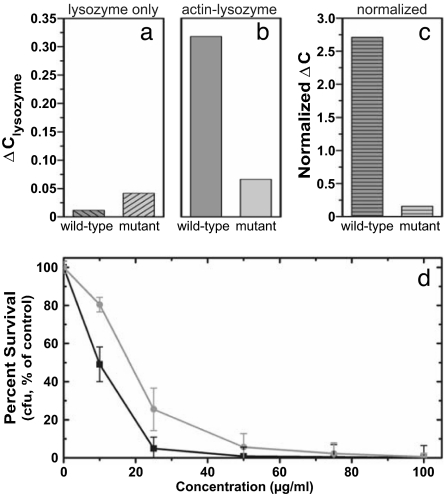
Fig. 6.
Microequilibrium dialysis experiments indicate that charge-reduced lysozyme is drastically less susceptible to actin-induced sequestration than WT lysozyme is, which is measured by ΔClysozyme = (Cexperimental − Ctheoretical)/Ctheoretical, where Ctheoretical is the expected concentration of a fully dialyzed solution (0.36 mg/ml) or of the initial lysozyme concentration (0.72 mg/ml). ΔClysozyme is shown for dialysis of 0.72 mg/ml lysozyme solutions in the absence of actin (a) and in the presence of 1.4 mg/ml F-actin (b). (c) Results for the actin–lysozyme system normalized by those for the lysozyme-only system. This normalization corrects for potential differential adhesion of the different lysozyme to the dialysis membrane and shows a 17-fold decrease in sequestration for the mutant (light gray) relative to the WT (dark gray). (d) Results from antibacterial killing assays showing the percentage survival of PAO1 bacteria in the presence of increasing WT lysozyme (black line) and double-mutant lysozyme (gray line) concentrations. These results indicate that, despite structural changes, the double mutant retains most of the WT antibacterial activity.