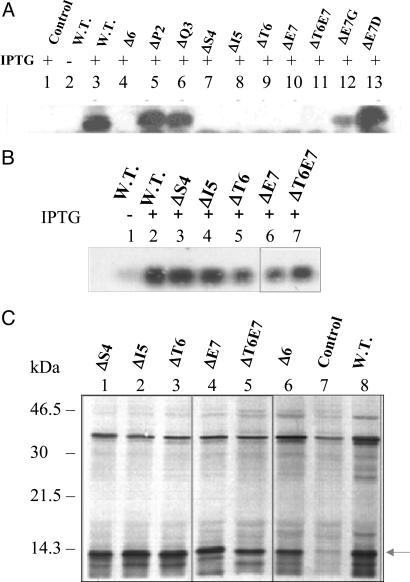
Fig. 5.
Analysis of expression of other LTB mutants. (A) Immunoblot analysis of expression. The E. coli DH5α cells harboring various mutant plasmids were induced at A600 = 0.4 for 5 h. The total cell extracts from different cultures were analyzed by Western blotting using anti-LTB polyclonal antibodies. Lane 1, control pMMB66EH; lanes 2 and 3, wild-type (W.T.) pMMB68; lane 4, pLTBΔ6; lane 5, pLTBΔP2; lane 6, pLTBΔQ3; lane 7, pLTBΔS4; lane 8, pLTBΔI5; lane 9, pLTBΔT6; lane 10, pLTBΔE7; lane 11, pLTBΔT6E7; lane 12, pLTBE7G; lane 13, pLTBT7D. + and − denote cells grown in the presence and absence of IPTG, respectively. (B) Northern blot analysis of LTB mutants. E. coli DH5α cells harboring the respective plasmids were induced for 5 h. Total RNA was isolated and subjected to Northern blot analysis using a radiolabeled ltb gene probe. Lanes 1 and 2, W.T. pMMB68; lane 3, pLTBΔS4; lane 4, pLTBΔI5; lane 5, pLTBΔT6; lane 6, pLTBΔE7; lane 7, pLTBΔT6E7. + and − denote cells grown in the presence and absence of IPTG, respectively. (C) In vitro translation of LTB mutants. E. coli S30 extract for linear template was used for in vitro synthesis. Protein labeling was carried out in the presence of 1.85 × 106 Bq of [35S] methionine. Equimolar concentrations of control and deletion plasmid DNA were used. The translated product was acetone-precipitated, separated on SDS/15% PAGE, and visualized by autoradiography. Lane 1, pLTBΔS4; lane 2, pLTBΔI5; lane 3, pLTBΔT6; lane 4, pLTBΔE7; lane 5, pLTBΔT6E7; lane 6, pLTBΔ6; lane 7, pMMB66EH (negative control); lane 8, pMMB68 (W.T.). The translated product is shown by an arrow.