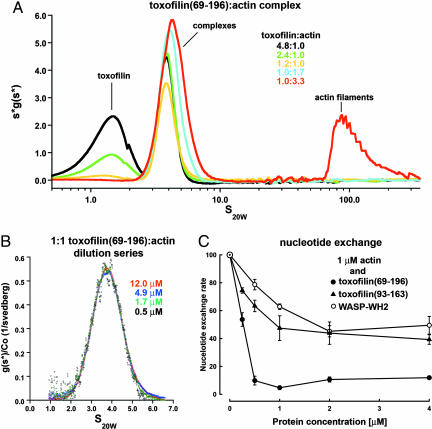
Fig. 2.
Analysis of the toxofilin69–196–actin complex in solution. (A) AUC s*g(s*) plot prepared with the program SEDVIEW (37) of toxofilin69–196:actin mixtures (x axis, sedimentation coefficients on a logarithmic scale in svedbergs at 20°C in water; y axis, sedimentation distribution function multiplied by s*). Each curve represents a combined series of s*g(s*) plots, including all of the scans of a single sedimentation velocity experiment. The area under each curve is proportional to the mass concentration of the sedimenting species. Note that actin filaments appear only in the 1:3.3 mixture (red curve, peak between 65 and 350 S), whereas excess toxofilin69–196 (peaks centered at 1.5 S) appears only in the 4.8:1, 2.4:1, and 1.2:1 mixtures. (B) g(s) plot produced with the program SEDANAL (35) of a dilution series of a 1:1 toxofilin69–196:actin mixture (x axis, sedimentation coefficient; y axis, sedimentation distribution function divided by the loading concentration). The peak corresponding to the 1:1 complex at 3.85 S is stable with dilution and appears alone but presents a minor shoulder at higher S values. This shoulder becomes part of a wider reaction boundary in the 1:1.7 and 1:3.3 mixtures. (C) Inhibition of nucleotide exchange on actin by toxofilin. ε-ATP–actin (1 μM) was mixed with varying concentrations of toxofilin69–196 (●), toxofilin93–163 (▴), or the WH2 of WASP (○), and the fluorescence decay was monitored after addition of 1.1 mM ATP. Note that steady ≈90% inhibition is attained at a toxofilin69–196:actin ratio of 1:2.