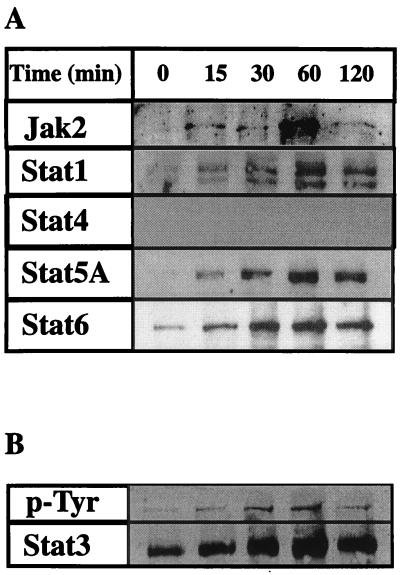
Figure 4.
Tyrosine phosphorylation of STATs after Ang II treatment of rat primary cardiac cells. (A) Extracts from control and AngII-treated cell at different time points were immunoprecipitated with anti-phosphotyrosine antibodies (4G10). After isolating the tyrosine phosphorylated protein by agarose-protein-A treatment, the proteins were separated in a 7% SDS/polyacrylamide gel and electroblotted on to nitrocellulose membrane. Each membrane was tested against polyclonal anti-Jak2, -STAT1, -STAT3, -STAT4, -STAT5A, and -STAT6 antibodies (Santa Cruz Biotechnology). (B) To evaluate that the increased phosphorylation activity of STAT proteins was due to AngII treatment, cell extracts from control and AngII-treated cardiomyocyte were immunoprecipitated with anti-STAT3, blotted against anti-phosphotyrosine (4G10), and then reblotted against STAT3. As observed equal amount of STAT3 is present at each time point, providing a loading control for the protein.