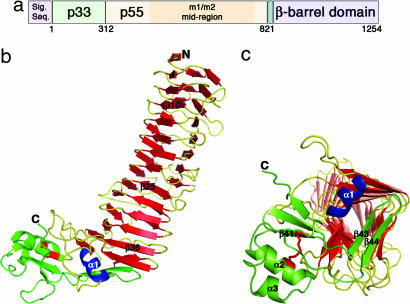
Fig. 1.
VacA structure. (a) The vacA gene encodes a 140-kDa protoxin. The mature 88-kDa VacA toxin contains two domains, designated p33 and p55. The midregion sequence that defines type m1 and m2 forms of VacA is located within p55. (b) The VacA p55 fragment adopts a β-helix structure that is composed of three parallel β-sheets (red) connected by loops of varying length and structure (yellow). The α-helix in blue (α1) is contained within one of these loops but is highlighted in blue to show how it caps the end of the β-helix. The C-terminal domain (green) has a mixture of α/β secondary structure elements and contains a disulfide bond (red), not previously observed in an autotransporter passenger domain structure. A few of the secondary structural elements are labeled to serve as landmarks and correlate to the sequences depicted in SI Fig. 7. (c) The C terminus of the β-helix is capped by a β-hairpin from the C-terminal domain (green) and the α1 α-helix (blue) located in one of the long β-helix loops. This view represents a rotation of the molecule in b by ≈90° into the plane of the page.