Abstract
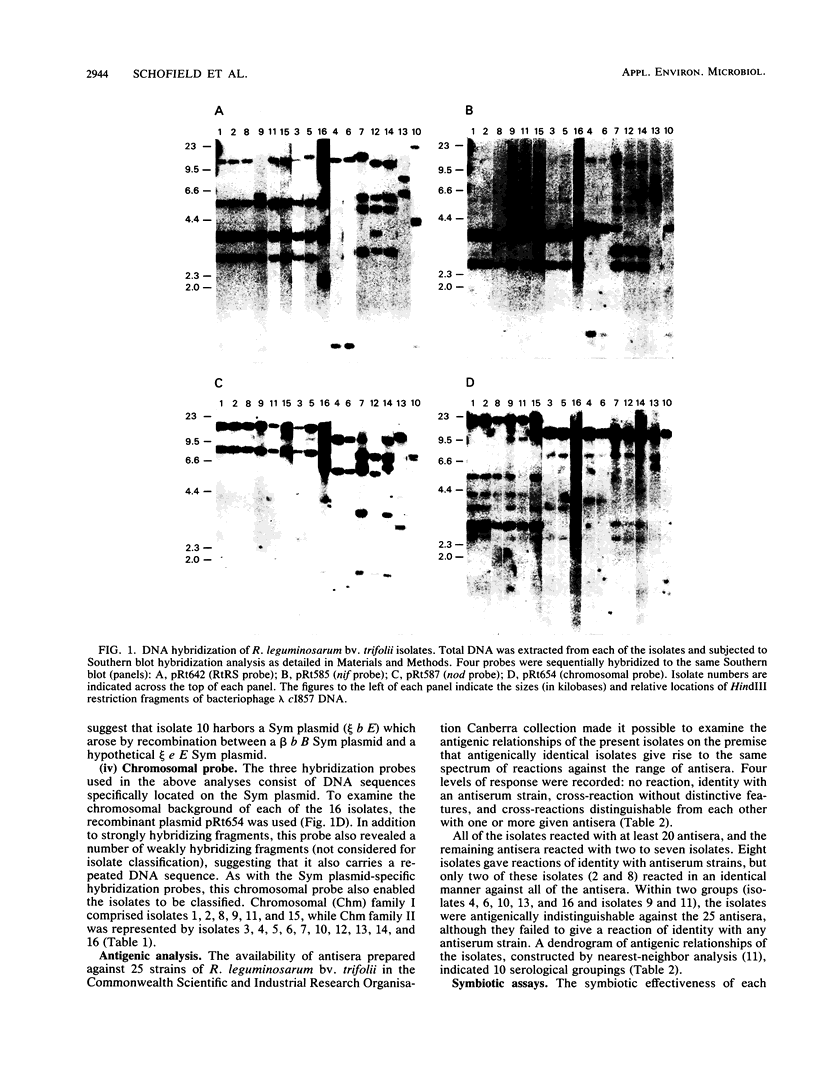
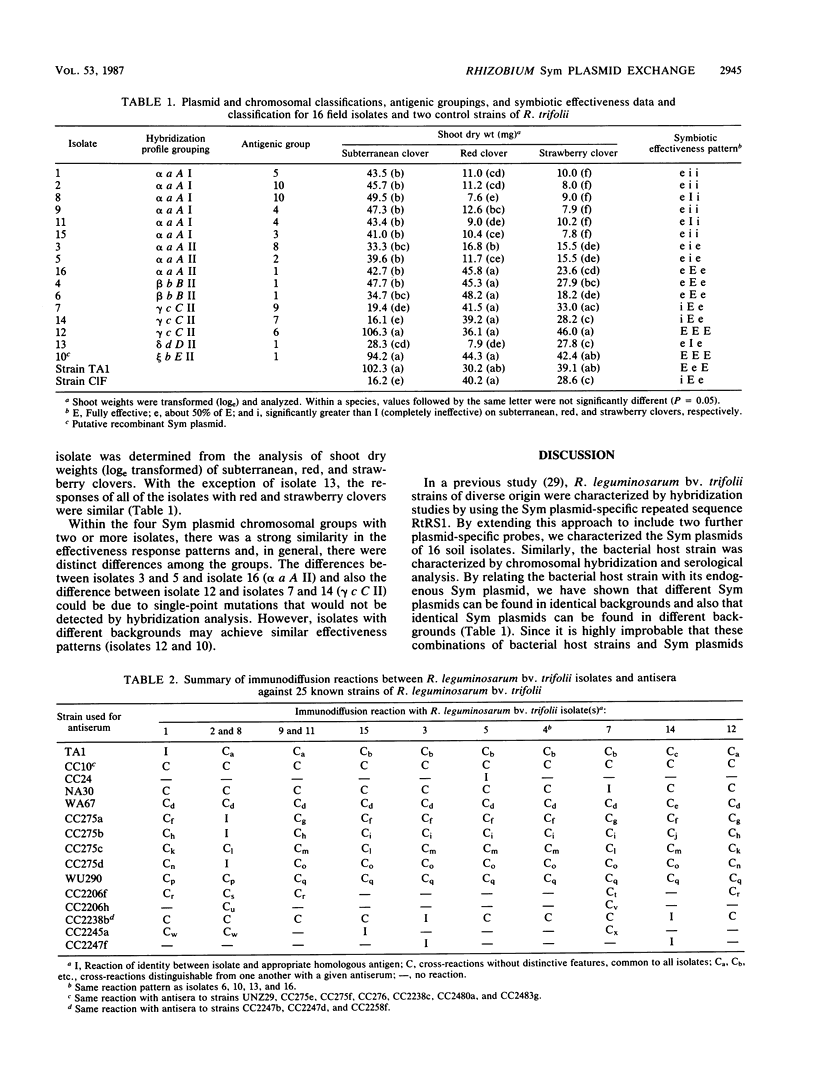
A soil population of 16 Rhizobium leguminosarum bv. trifolii isolates was characterized by using three Sym (for symbiotic) plasmid-specific DNA hybridization probes: (i) an R. leguminosarum bv. trifolii-specific, repeated-sequence probe; (ii) a nifHDK gene probe, and (iii) a nod gene probe. A predominant Sym plasmid family was identified among the isolates. Three other unrelated Sym plasmid families were also identified. The isolates were also classified either by using a chromosomal DNA hybridization probe or by serological relatedness to 25 different R. leguminosarum bv. trifolii antisera. With either method, it was possible to group the 16 soil isolates into identical or related families. However, the correlation between the two techniques was not high. Irrespective of the means used to classify the bacterial host strain, it was possible to identify the same Sym plasmids in unrelated strains, as well as unrelated Sym plasmids in identical host strains. These data indicate that, within this soil population, there has been genetic exchange of Sym plasmids, and in one instance the hybridization pattern indicates that in vivo recombination of two different Sym plasmids may have occurred. Symbiotic effectiveness tests on red, strawberry, and subterranean clovers clearly differentiated the isolates. In general, the pattern of response was similar within groupings on the basis of Sym plasmid and chromosomal profiles but different between such groups.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Broughton W. J., Heycke N., Z A H. M., Pankhurst C. E. Plasmid-linked nif and "nod" genes in fast-growing rhizobia that nodulate Glycine max, Psophocarpus tetragonolobus, and Vigna unguiculata. Proc Natl Acad Sci U S A. 1984 May;81(10):3093–3097. doi: 10.1073/pnas.81.10.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A. H., Schubert K. R. Identification of a Rhizobium trifolii plasmid coding for nitrogen fixation and nodulation genes and its interaction with pJB5JI, a Rhizobium leguminosarum plasmid. J Bacteriol. 1983 Nov;156(2):592–599. doi: 10.1128/jb.156.2.592-599.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic M. A., Zurkowski W., Shine J., Rolfe B. G. Sym plasmid transfer to various symbiotic mutants of Rhizobium trifolii, R. leguminosarum, and R. meliloti. J Bacteriol. 1983 Dec;156(3):1035–1045. doi: 10.1128/jb.156.3.1035-1045.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Ma Q. S., Knight C. D., Hombrecher G., Johnston A. W. Cloning of the symbiotic region of Rhizobium leguminosarum: the nodulation genes are between the nitrogenase genes and a nifA-like gene. EMBO J. 1983;2(6):947–952. doi: 10.1002/j.1460-2075.1983.tb01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Ronson C. W., Astwood P. M., Downie J. A. Molecular cloning and genetic organization of C4-dicarboxylate transport genes from Rhizobium leguminosarum. J Bacteriol. 1984 Dec;160(3):903–909. doi: 10.1128/jb.160.3.903-909.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. R., Watson J. M. DNA sequence of Rhizobium trifolii nodulation genes reveals a reiterated and potentially regulatory sequence preceding nodABC and nodFE. Nucleic Acids Res. 1986 Apr 11;14(7):2891–2903. doi: 10.1093/nar/14.7.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotnicki M. L., Rolfe B. G. Transfer of nitrogen fixation genes from a bacterium with the characteristics of both Rhizobium and Agrobacterium. J Bacteriol. 1978 Feb;133(2):518–526. doi: 10.1128/jb.133.2.518-526.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfeld P. L., Seeburg P. H., Shine J. The human pro-opiomelanocortin gene: organization, sequence, and interspersion with repetitive DNA. DNA. 1982;1(2):133–143. doi: 10.1089/dna.1.1982.1.133. [DOI] [PubMed] [Google Scholar]