Figure 3.
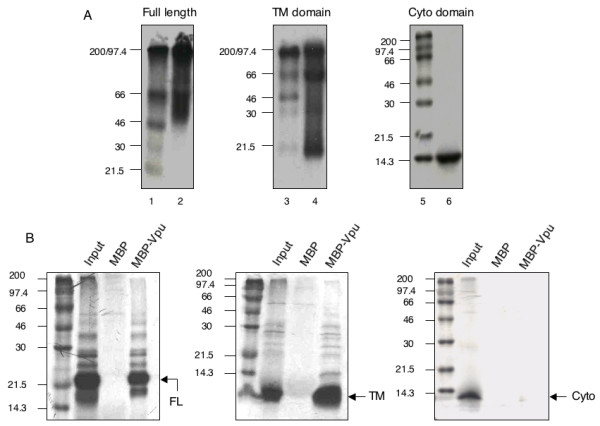
Vpu oligomerization based on gel electrophoretic and pull-down assays. The full-length R5-Vpu protein and its transmembrane and cytoplasmic domains were synthesized and labeled with 35S-methionine in a coupled in vitro transcription-translation system. (A) The proteins were analyzed on native polyacrylamide gels without heating or DTT treatment. Lanes 1,3 and 5, markers; lanes 2,4 and 6, Vpu full-length, TM domain and cytoplasmic domain, respectively. The molecular sizes (in kilodaltons) are indicated. (B) For pull-down assays, the 35S-labeled R5-Vpu proteins were synthesized in vitro and bound to amylose beads saturated with either the maltose binding protein (MBP)/R5-Vpu fusion protein or MBP alone as a control. The beads were washed, resuspended in loading dye buffer, boiled and the supernatants subjected to SDS-PAGE. The gels were dried and autoradiographed. Gels show the full-length (FL) R5-Vpu protein, or its transmembrane (TM) or cytoplasmic (cyto) domains, retained on the beads. Arrows indicate the full-length or truncated Vpu proteins.
