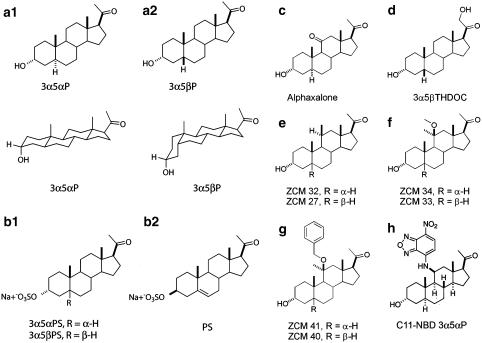
Figure 1.
Structures of types of GABA potentiating and GABA antagonistic neuroactive steroids used in this study. (a) 3α5αP and 3α5βP (allopregnanolone and pregnanolone) are GABAA receptor potentiators despite their structural differences. (b) Sulphated pregnane and pregnene steroids are effective non-competitive GABAA receptor antagonists. (c–h) Other structural modifications tolerated by potentiating steroids. (c) Alphaxalone is a synthetic anaesthetic steroid (Phillipps, 1975; Barker et al., 1987). (d) 3α5βTHDOC is a naturally occurring steroid (Majewska et al., 1986; Mellon et al., 2001). (e–h) Synthetic neuroactive steroids with substitutions at C11 are shown as ZCM 32, ZCM 34 and ZCM 41 (5α-reduced series) and ZCM 27, ZCM 33 and ZCM 40 (5β-reduced series). C11-NBD 3α5αP has a fluorescent substituent at carbon 11.