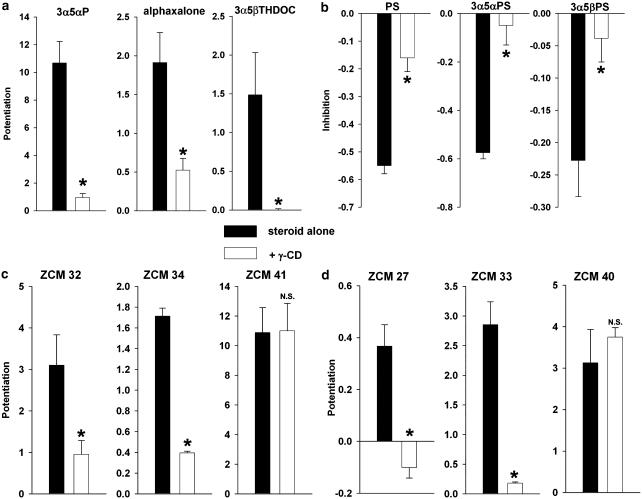
Figure 7.
Cyclodextrin effects on neuroactive steroid potentiation and inhibition. (a) Neuroactive steroid potentiators were used to modulate responses to 2 μM GABA, 3α5αP at 0.5 μM, alphaxalone at 0.5 μM and 3α5βTHDOC at 0.5 μM. Co-application with 0.5 mM γ-cyclodextrin (γ-CD) significantly blocked potentiation (n=4–13 for each experiment, asterisks indicate P<0.05 compared to steroid alone). (b) Neuroactive steroid inhibitors were used to antagonize responses to 30 μM GABA, PS at 1 μM, (3α,5α)-3-hydroxypregnan-20-one sulphate (3α5αPS) at 1 μM, and 1 μM (3α,5β)-3-hydroxypregnan-20-one sulphate (3α5βPS). Co-application with 0.5 mM γ-cyclodextrin significantly blocked inhibition. (c and d) The interaction of synthetic 5α- and 5β-reduced neuroactive steroids and cyclodextrins. (c) Synthetic 5α-reduced neuroactive steroids with small to large substitutions at C11 (ZCM 32<ZCM 34<ZCM 41) were applied at 0.25 μM with 2 μM GABA. Co-application with 0.5 mM γ-cyclodextrin blocked potentiation in steroids with the smaller substitution at C11 but failed to block potentiation of ZCM 41, which contains a large aromatic substitution at C11. (d) Synthetic 5β-reduced neuroactive steroids in the same series (ZCM 27<ZCM 33<ZCM 40). Cyclodextrin blocked potentiation of the smaller steroids but not that of the larger steroids. The mean potentiation was apparently reduced below zero in the case of ZCM 27, but this appeared to be due to nonspecific small rundown of the GABA response in several cells, and the depression below zero was not statistically significant (P>0.05). In (b, c and d) n=8, asterisks indicate P<0.05 compared with steroid alone, NS indicates not significant.