Abstract
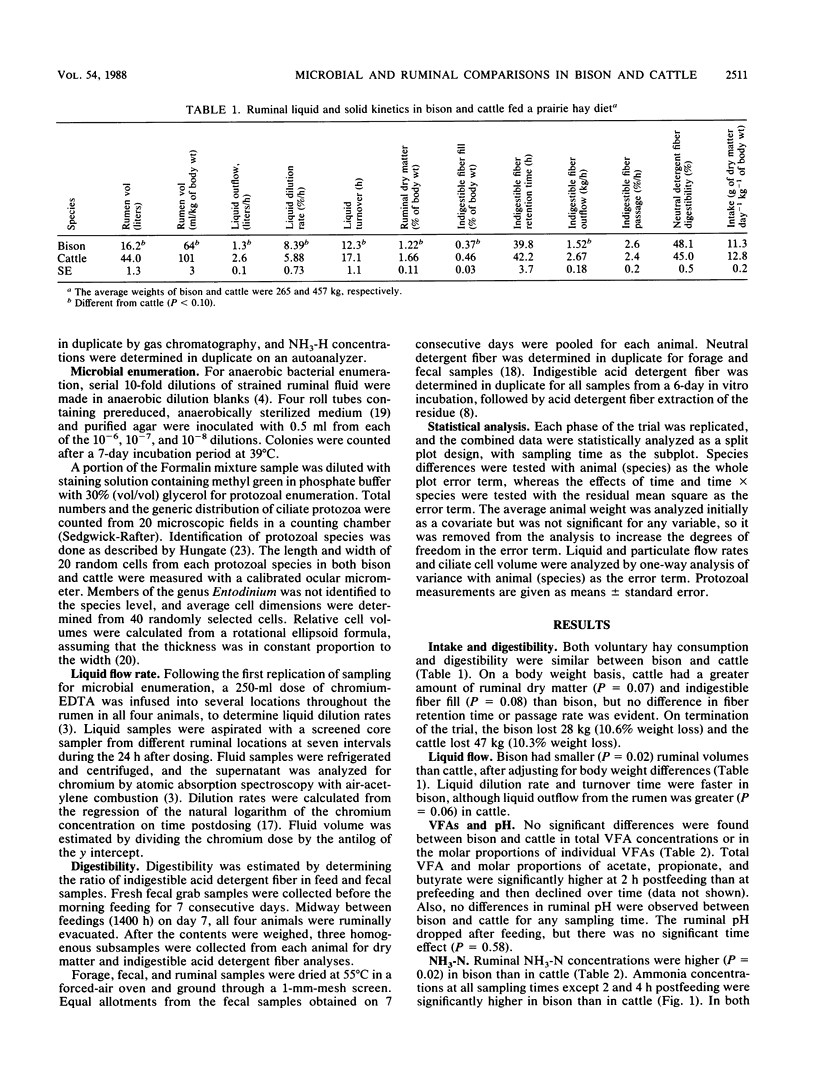
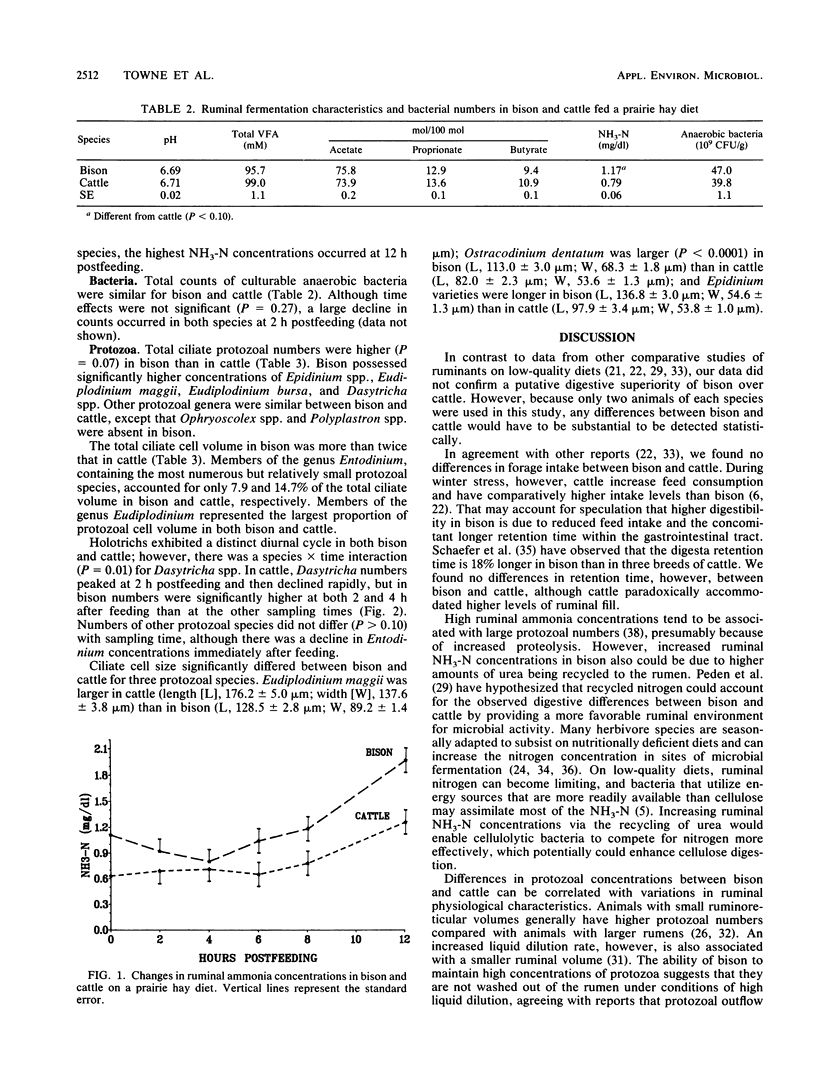
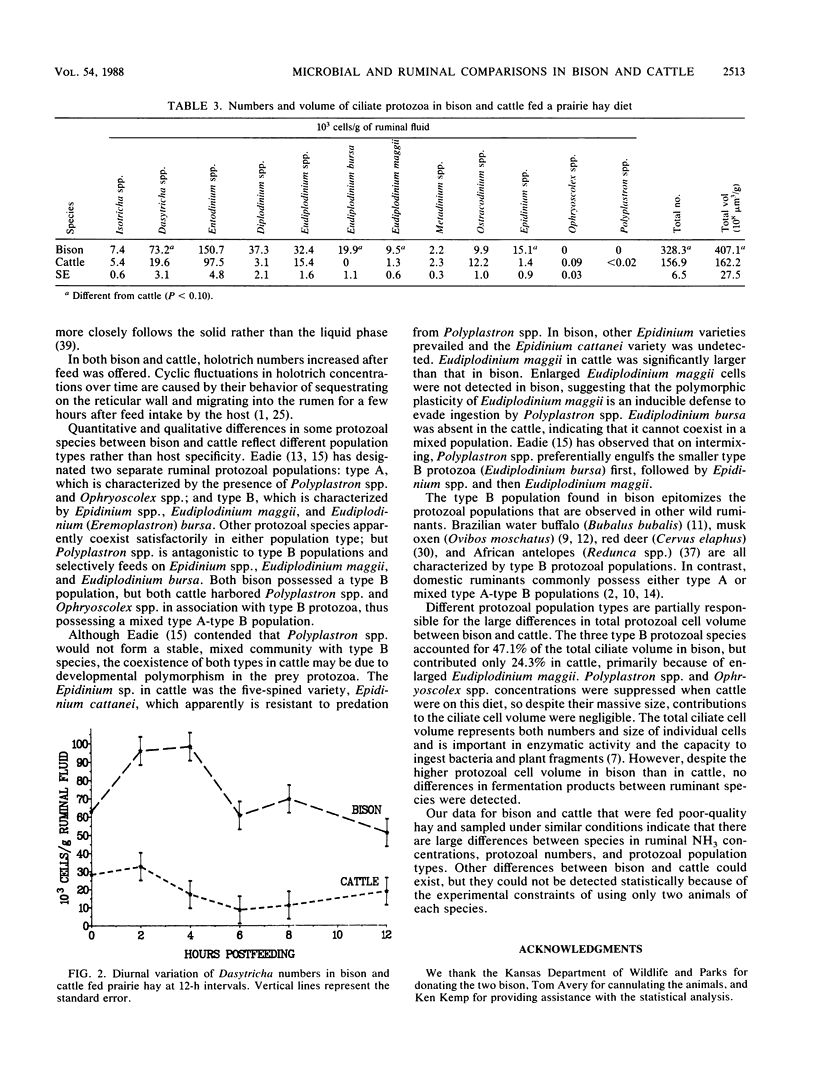
Ruminal microbial populations, fermentation characteristics, digestibility, and liquid flow rates in two ruminally cannulated bison and two ruminally cannulated Hereford steers fed a prairie hay diet were compared. No significant differences in anaerobic bacterial counts, volatile fatty acid concentrations, or ruminal pHs were evident between bison and cattle. Also, no significant differences in neutral detergent fiber digestibility, indigestible fiber retention time, or intake were detected between bison and cattle, although cattle had higher levels (P less than 0.08) of ruminal dry matter and indigestible fiber than bison. Bison had a smaller (P = .02) ruminoreticular volume, faster liquid dilution rates, and faster liquid turnover times than cattle. The average ruminal ammonia nitrogen concentration was higher (P = 0.02) in bison (1.17 mg/dl) than in cattle (0.79 mg/dl). Total ciliate protozoal counts and cell volume were greater (P = 0.07) in bison (32.8 x 10(4)/g and 407.1 x 10(-4) ml/g, respectively) than in cattle (15.7 x 10(4)/g and 162.2 x 10(-4) ml/g, respectively). Bison harbored higher (P less than 0.02) numbers of Dasytricha spp., Eudiplodinium maggii, Eudiplodinium bursa, and Epidinium spp. than cattle and possessed a type B protozoan population. The cattle possessed a mixed type A-type B population that was characterized by Ophryoscolex spp. and Polyplastron spp. in association with low concentrations of Epidinium spp. and Eudiplodinium maggii.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Iriki T., Tobe N., Shibui H. Sequestration of holotrich protozoa in the reticulo-rumen of cattle. Appl Environ Microbiol. 1981 Mar;41(3):758–765. doi: 10.1128/aem.41.3.758-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Akkada A. R., Bartley E. E., Fina L. R. Ciliate protozoa in the rumen of the lactating cow. J Dairy Sci. 1969 Jul;52(7):1088–1091. doi: 10.3168/jds.S0022-0302(69)86697-X. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Studies on the Nitrogen Requirements of Some Ruminal Cellulolytic Bacteria. Appl Microbiol. 1961 Mar;9(2):96–103. doi: 10.1128/am.9.2.96-103.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. T., Ulyatt M. J., John A. Variation in Numbers and Mass of Ciliate Protozoa in the Rumens of Sheep Fed Chaffed Alfalfa (Medicago sativa). Appl Environ Microbiol. 1982 May;43(5):1201–1204. doi: 10.1128/aem.43.5.1201-1204.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Ciliate protozoa in the rumen of Brazilian water buffalo, Bubalus bubalis Linnaeus. J Protozool. 1979 Nov;26(4):536–544. doi: 10.1111/j.1550-7408.1979.tb04191.x. [DOI] [PubMed] [Google Scholar]
- Eadie J. M. Studies on the ecology of certain rumen ciliate protozoa. J Gen Microbiol. 1967 Nov;49(2):175–194. doi: 10.1099/00221287-49-2-175. [DOI] [PubMed] [Google Scholar]
- Grubb J. A., Dehority B. A. Variation in colony counts of total viable anaerobic rumen bacteria as influenced by media and cultural methods. Appl Environ Microbiol. 1976 Feb;31(2):262–267. doi: 10.1128/aem.31.2.262-267.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. R., Drone P. E., Jr, Woodford S. T. Factors stimulating migration of holotrich protozoa into the rumen. Appl Environ Microbiol. 1985 May;49(5):1329–1331. doi: 10.1128/aem.49.5.1329-1331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour A. M., Abou Akkada A. R., el-Shazly K., Naga M. A., Borhami B. E., Abaza M. A. Effect of increased levels of urea in the diet on ruminal protozoal counts in four ruminant species. J Anim Sci. 1979 Nov;49(5):1300–1305. doi: 10.2527/jas1979.4951300x. [DOI] [PubMed] [Google Scholar]
- Orpin C. G., Mathiesen S. D., Greenwood Y., Blix A. S. Seasonal changes in the ruminal microflora of the high-arctic Svalbard reindeer (Rangifer tarandus platyrhynchus). Appl Environ Microbiol. 1985 Jul;50(1):144–151. doi: 10.1128/aem.50.1.144-151.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson H. A. Rumen microorganisms in buffalo from southern utah. Appl Microbiol. 1967 Nov;15(6):1450–1451. doi: 10.1128/am.15.6.1450-1451.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins C. T., Prior R. L., Moen A. N., Visek W. J. Nitrogen metabolism of white-tailed deer. J Anim Sci. 1974 Jan;38(1):186–191. doi: 10.2527/jas1974.381186x. [DOI] [PubMed] [Google Scholar]
- Schaefer A. L., Young B. A., Chimwano A. M. Ration digestion and retention times of digesta in domestic cattle (Bos taurus), American bison (Bison bison), and Tibetan yak (Bos grunniens). Can J Zool. 1978 Nov;56(11):2355–2358. doi: 10.1139/z78-318. [DOI] [PubMed] [Google Scholar]
- Veira D. M., Ivan M., Jui P. Y. Rumen ciliate protozoa: effects on digestion in the stomach of sheep. J Dairy Sci. 1983 May;66(5):1015–1022. doi: 10.3168/jds.S0022-0302(83)81896-7. [DOI] [PubMed] [Google Scholar]
- Weller R. A., Pilgrim A. F. Passage of protozoa and volatile fatty acids from the rumen of the sheep and from a continuous in vitro fermentation system. Br J Nutr. 1974 Sep;32(2):341–351. doi: 10.1079/bjn19740087. [DOI] [PubMed] [Google Scholar]


