4.
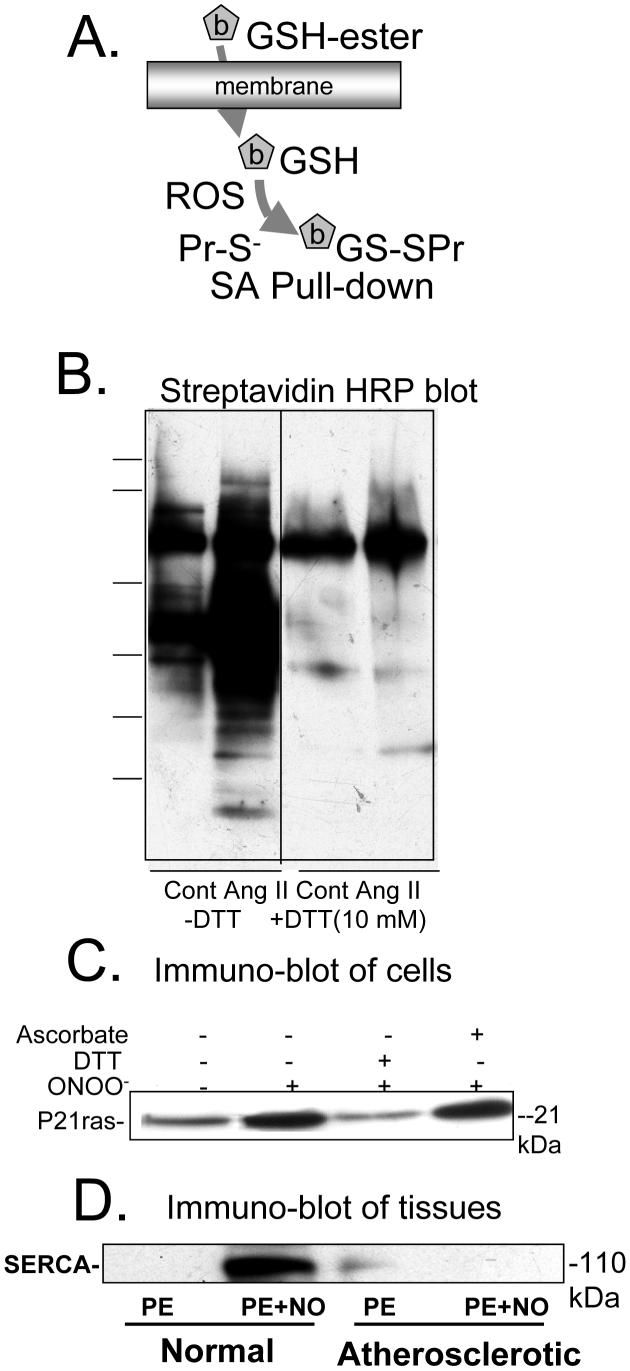
Biotin-tagged glutathione labeling of proteins in cells and tissues exposed to RNS/ROS. Biotin-tagged GSH is introduced into cells or tissues with the ester, which is cleaved by cellular esterases. RNS/ROS result in binding of the GSH adduct predominantly to protein thiolate anions. B. Streptavidin-HRP blot of lysates of rat aortic smooth muscle cells pre-labeled with biotin-tagged GSH and exposed to angiotensin II (Ang II). When cells were lysed in the absence of dithiothreitol (-DTT), multiple proteins were labeled in control cells (Cont), and the amount of labeling increased in cells exposed to Ang II. When the lysate was treated with DTT (10 mM, +DTT), much less streptavidin-HRP stained protein was detected. The remaining bands in lysates treated with DTT and that were unchanged by Ang II treatment are likely endogenous biotinylated proteins. From reference [7]. C. Immuno-blot for p21ras of avidin pull-down from lysates of bovine aortic endothelial cells prelabeled with biotin-tagged GSH and treated with ONOO- (100 μM, 5 min). ONOO- increased p21ras in the avidin affinity pull-down. DTT (1 mM), but not ascorbate (1 mM), added to the same cell lysate reduced the disulfide bond, cleaving the majority of the adduct from the protein. From [9]. D. Immuno-blot for SERCA of avidin pull-down from lysates of intact rabbit aortic rings pre-labeled with biotin-tagged GSH prior to exposure to nitric oxide (NO, 10 μM) during physiological contraction to phenylephrine. In normal rabbit aorta, NO increased the amount of SERCA labeled with the biotin-tagged GSH, whereas it did not increase labeling in atherosclerotic rabbit aorta in which it was demonstrated that the thiolate anionic site of binding was irreversibly oxidized. From [8].