6.
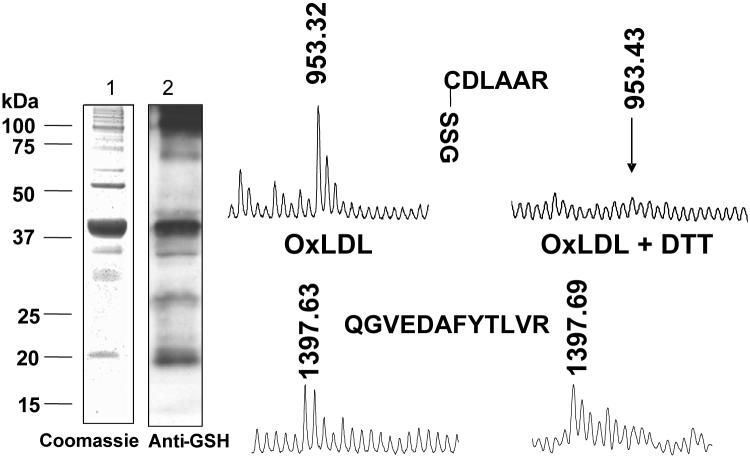
Detection by mass spectrometry of S-glutathione adducts on p21ras. Bovine aortic endothelial cells were treated with oxidized low density lipoproteins (LDL, 100 μg/mL, 1 h) and p21ras was immunoprecipitated from 1 mg of cell lysate protein under non-reducing conditions. p21ras was separated by SDS PAGE. Gels at left show immuno-precipitate stained with Coomassie blue (lane 1) and immuno-blot with anti-GSH antibody (lane 2) showing a prominent 21 kDa band. The protein in this band was digested in-gel with trypsin, and peptides were recovered and subjected to MALDI-TOF mass spectrometry. A peak in the mass spectrum corresponding to a tryptic peptide with a mass (953.32 Da) consistent with S-glutathiolated cysteine-118 (CDLAAR) was present, whose mass was 305 Da greater than the predicted mass of the peptide. When the lysate was treated with DTT, the mass spectrum peak was undetectable (right). In the same sample, a peak (1397.63 Da) representing another p21ras peptide that does not contain cysteine (QGVEDAFYTLVR, lower) appeared in the spectra of both non-reduced and reduced samples. From reference [23].