Abstract
Immunogenicity profiles of recombinant therapeutic proteins are important to understand because antibodies raised against these molecules may have important clinical sequelae. The purpose of the present study was to demonstrate that a flow cytometric bead array could be used to detect clinically relevant antibodies with specificity to such therapeutics. We chose to evaluate well-characterized specimens from persons treated with epoetin alfa that developed antibody-mediated pure red blood cell aplasia as a means to demonstrate the utility of this platform. Our data show that this assay is capable of detecting anti-epoetin alfa antibodies with a relative antibody concentration of 50 ng/ml, where 25 of 25 sera spiked with antibodies at this concentration scored positive. Moreover, the assay was designed to include positive and negative control beads for each specimen that is processed to ensure the specificity of the signal when detected. Measurement of interassay precision supports quantitative estimates of relative antibody concentrations in the range of 313 to 5,000 ng/ml, where the percent coefficient of variation did not exceed 20%. With respect to clinical specimens, antibodies with specificity for epoetin alfa could be easily detected in a set of specimens from persons with pure red blood cell aplasia that had prior exposure to the EPREX brand of recombinant epoetin alfa. Further development and validation of this approach may facilitate successful widespread application of the method for detection of anti-epoetin alfa antibodies, as well as antibodies directed against other recombinant therapeutic proteins.
Immune responses that occur in vivo during administration of a therapeutic protein can induce clinically significant adverse events, including neutralization of both the therapeutic and its endogenous counterpart. Although all antibody isotypes may have this property, manifestations appear to emerge after the class switch to the immunoglobulin G (IgG) isotype (11, 17). Clinical management of persons with antibody-neutralized endogenous proteins is not straightforward and most commonly involves the cessation of (drug) administration with supportive care on a case-by-case basis until the circulating concentration of antibodies decreases to nonpathological levels (1). The prolonged half-life of antibodies and plasma cells makes this a long-term issue for the patient.
A prototypical example of the ability of a recombinant therapeutic protein to raise cross-reactive, neutralizing antibodies is provided by patients that developed antibody-mediated pure red blood cell aplasia during treatment with recombinant epoetin alfa manufactured and sold outside of the United States. This protein was linked to the induction of neutralizing IgG antibodies and the development of pure red cell aplasia (PRCA) in more than 200 patients since 1998 (1), with the majority of cases attributed to the EPREX brand of recombinant epoetin alfa. Even though the manufacturer of EPREX has since taken action to potentially remediate this situation (3, 4), there is no consensus that the problem has in fact been solved (19, 21). Moreover, the emergence of biosimilar therapeutics has been considered by some to increase the odds that a clinically significant immunogenicity issue will occur in the future (6, 13).
Currently, each drug manufacturer is responsible for internal development and execution of antibody detection and surveillance programs. The assays that are offered vary widely (12, 24), each with individual performance characteristics (22), and there is no formal certification program to ensure that a given assay is suitable for clinical diagnoses. Despite the longstanding commercial success of human recombinant erythropoiesis stimulating proteins, the lack of a standardized clinical assay to provide a differential diagnosis of this adverse event places patients at unnecessary risk.
The clear need for the biotechnology industry to apply robust, harmonized assays in this setting motivated our laboratory to design a multiplex immunoassay platform. This approach is gaining popularity in a variety of settings, including immunogenicity assessments for persons treated with recombinant granulocyte colony-stimulating factor (2), new approaches to monitor for vaccine responsiveness in clinical trials (7), and measurement of autoantibodies in the clinic (20). Among the many advantages to multiplexing in this setting, the ability to include positive and negative controls in each specimen that is analyzed may be regarded as a particular strength of this approach, as illustrated by the experiments presented here. It is ultimately envisioned that the cytometric bead array could serve a diagnostic role that could complement more extensive testing and characterization of the antibody response, including the formal demonstration of neutralizing potential in a cell-based assay (26) (by the manufacturer of the therapeutic agent).
MATERIALS AND METHODS
Serum specimens.
Serum from normal donors was purchased from Bioreclamation, Inc. (Hicksville, NY). Serum from persons who developed PRCA subsequent to epoetin alfa therapy was provided to Amgen Clinical Immunology Laboratories by Nicole Casadevall (Hôpital de l'Hôtel-Dieu, Paris, France); these specimens were collected in compliance with institutional policies and had been previously characterized in a variety of cell-based and immunoassay platforms (5, 19). All specimens were stored frozen at −20°C until processing.
Preparation of beads and serum samples for cytometric bead immunoassay.
Beads (Becton Dickinson) of distinct and nonoverlapping fluorescence emission intensities were covalently coupled with epoetin alfa (Amgen, Inc., Thousand Oaks, CA), sperm whale myoglobin (Sigma-Aldrich, St. Louis, MO), or human IgG (Becton Dickinson) via standard amine chemistry (10). A fourth “blank” bead was left unconjugated and served as a reference negative control bead (in addition to the sperm whale myoglobin bead). The beads were identical to those used in the commercially available cytometric bead array assays marketed by Becton Dickinson and are hard dyed with a water-insoluble dye that is excited by the 488-nm laser source (with emission at 576 nm). To perform the assay, beads were vortexed vigorously, and then 15 μl of each of the four bead types was combined to comprise the bead master mixture, 50 μl of which was added to each multiplex assay tube.
The assay was set up as depicted in Fig. 1 and followed the commercially available cytometric bead array platform protocol (16). Briefly, 50 μl of bead mixture was combined with 50 μl of serum (diluted to a 1% [vol/vol] concentration), vortexed, and then incubated for 2.5 h at room temperature. Samples were washed by the addition of 1 ml of wash buffer and then centrifuged at 200 × g for 5 min. The supernatant was aspirated, and then 50 μl of phycoerythrin detection reagent was added to each tube, vortexed, and then incubated for 30 min at room temperature. Samples were then washed once as described above, resuspended in 300 μl of wash buffer, and analyzed on a flow cytometer.
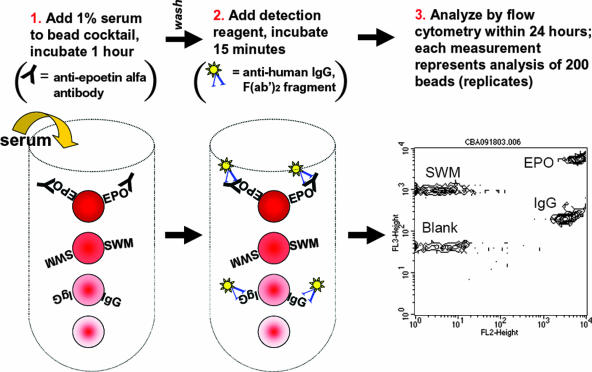
FIG. 1.
Flow cytometric bead immunoassay for anti-epoetin alfa antibodies. This multiplexed assay was designed with 4 beads of distinct fluorescence intensity that had epoetin alfa (EPO), sperm whale myoglobin (SWM; a negative control), or human IgG (IgG; a positive control) covalently attached to their surfaces by standard amine chemistry. A fourth bead was left unconjugated and served as a reference bead control. The assay involved incubation with 1% serum, followed by addition of a fluorescent detection reagent [phycoerythrin-conjugated anti-human IgG, F(ab′)2] and analysis on a standard flow cytometer. The intensity of fluorescence is therefore proportional to the amount of serum antibody captured by each of the respective beads. The address for each bead corresponds to the position in the diagram and is labeled as such in the rightmost dot plot from the flow cytometer.
On the day of the assay, the serum specimens were thawed at ambient temperature and diluted to 1% (vol/vol) in phosphate-buffered saline. For spike recovery experiments and serial dilution curves of the positive control antibody, the sera were initially diluted in pooled normal human serum and then diluted to a 1% concentration in phosphate-buffered saline for analysis.
Specimens were analyzed in accord with a conventional cytometric bead assay for cytokine detection (16) by using a FACSCalibur flow cytometer (Becton Dickinson). BD CellQuest Pro software was used in conjunction with the cytometric bead array instrument setup template and cytometer setup beads to adjust cytometer settings to correct baseline values. Briefly, cytometer setup beads adsorbed with fluorescein isothiocyanate- or phycoerythrin-positive control detector reagents were used to adjust the side scatter, forward scatter, FL1, FL2, and FL3 photomultiplier tube settings such that each of the four bead types clustered within the first decade of fluorescence. Analysis of a human negative control serum was used to visually verify that photomultiplier tube settings and compensation were correct. Upon analysis of raw data, the median fluorescence intensity (MFI) of each bead cluster was quantified. Where indicated in the figures, antibody concentrations were extrapolated relative to a standard curve created by serial dilution of the human positive control antibody. The concentrations of this antibody were expressed relative to rabbit polyclonal affinity-purified antisera (Amgen, Inc.) (14), since a human anti-epoetin alfa antibody calibrator is not available.
RESULTS
To begin validation of this assay system, we initially tested a serum specimen from a patient diagnosed with antibody-mediated PRCA that was initially included in a report from Casadevall et al. (5) and later characterized in our laboratory (22). Assessment by the cytometric bead array assay demonstrated a specific reactivity to the epoetin alfa bead with a signal-to-noise ratio of >1:1,000 (Fig. 1). The performance of positive and negative control beads was evident; high-level reactivity to the IgG bead was observed in conjunction with minimal reactivity to the sperm whale myoglobin (negative control) bead or the blank bead.
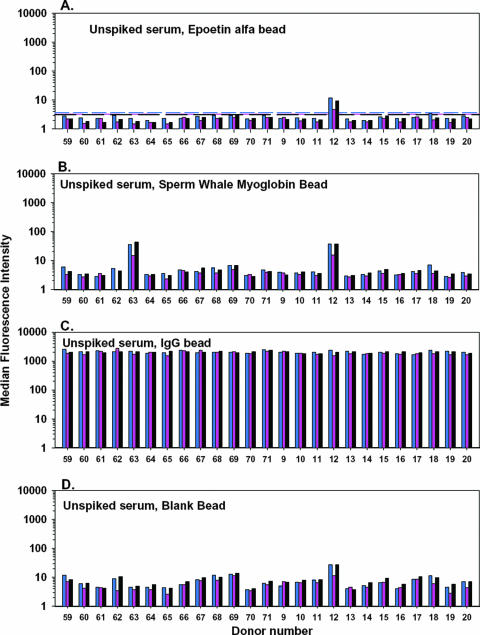
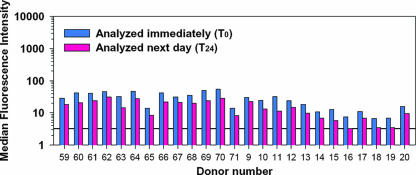
To provide validation data for the positive and negative control beads, commercially purchased sera from 25 individual donors was evaluated in three independent experiments (Fig. 2). The reactivity profiles of these sera reflected the absence of anti-epoetin alfa antibodies and allowed for calculation of a reactivity threshold from the 25 negative specimens. Here, the mean plus three standard deviations from each experimental run were calculated as an analytical cutoff, with concordance between runs. The serum from one donor (donor 12) exhibited binding to the epoetin alfa bead in excess of this threshold. The serum also appeared to be reactive to sperm whale myoglobin (Fig. 2B) and exhibited the highest level of binding to blank bead negative controls (Fig. 2D) of the 25 specimens included in the experiment. Because of the nonspecific binding profile of this specimen, it was excluded from the threshold calculations as an outlier. A second donor (donor 63) had a noteworthy reactivity to sperm whale myoglobin. This may reflect a bona fide cross-reactive antibody, since humans are known to have circulating anti-myoglobin antibodies (8, 9), and cross-reactivity between anti-myoglobin antibodies and myoglobins from a variety of species is not unusual (25).
FIG. 2.
Characterization of background binding of human serum IgG in the flow cytometric bead immunoassay. In this experiment, sera from 25 persons without prior exposure to an erythropoietic agent were tested to determine the background binding to each bead. The blue, pink, and black bars represent data from three independent experiments. The assay cutoffs, described as the mean plus the standard deviations, are displayed for each assay run as corresponding, color-matched dashed lines. See the text for a detailed discussion of the data.
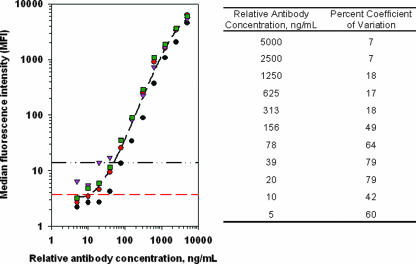
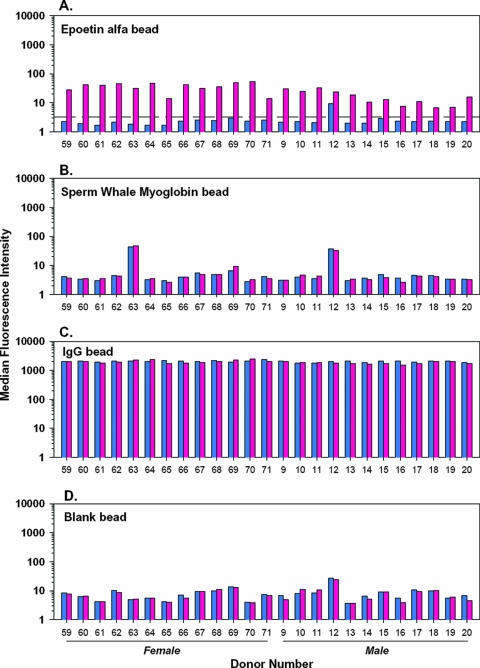
Additional experiments in this validation exercise included serial dilution of the human positive control serum (Fig. 3), followed by a spike recovery experiment in the samples from 25 normal individuals (Fig. 4). The serial dilution data (Fig. 3) represented four independent experiments and predicted that a relative anti-epoetin antibody concentration in the low nanograms-per-milliliter range could be reliably detected in this assay system. On the basis of this observation, a relative antibody concentration of 50.0 ng/ml was arbitrarily chosen and spiked into the 25 antibody-negative serum specimens. A positive signal was generated in 25 of 25 cases (100%; Fig. 4), establishing a limit of detection of 50 ng/ml. This falls well within the industry guidance range regarding the sensitivity of these types of immunoassays for clinical studies (250 to 500 ng/ml) (15). Further analysis of assay performance showed that the percent coefficient of variation for relative antibody concentrations from 50 to 313 ng/ml (Fig. 3, right side) did not meet industry guidance of 20 to 25% (15) for quantitative estimates of antibody concentrations. Relative antibody concentrations above 313 ng/ml were, however, characterized within acceptable limits (≤18% coefficient of variation) for quantitative estimates. Finally, it should be noted that addition of the anti-epoetin antibody in Fig. 4 had no impact on signal generated from the positive or negative control beads, speaking to the specificity of the assay platform and overall reliability of assay performance.
FIG. 3.
Serial dilution of a human serum specimen that was positive for anti-epoetin alfa antibodies. Serial dilution of this specimen on four independent experiments (coded by unique symbols) demonstrated a dose-response relationship between anti-epoetin antibody concentration and signal generated from the flow cytometer. The average regression line (black dashed line calculated in Sigmaplot version 8.0) from these four experiments is plotted for reference. The horizontal red dashed line indicates the mean plus three standard deviations from the negative control specimens; the horizontal black dashed/dotted line indicates where the 50-ng/ml concentration falls. This relative antibody concentration was subsequently used to document the sensitivity of the assay (Fig. 4).
FIG. 4.
Demonstration that the flow cytometric bead immunoassay is of adequate sensitivity for clinical application. In this experiment, the positive control specimen from Fig. 3 was spiked into the 25 serum specimens studied for background binding in Fig. 2 at a final relative antibody concentration of 50 ng/ml. A signal in excess of the mean plus three standard deviations of the unspiked specimens was demonstrated for all specimens. The signal was specific to the bead coupled to epoetin alfa; an increase in signal was not apparent in any of the other three beads in the multiplexed array.
Although not envisioned as a normal practice for this immunoassay, samples from the spike recovery experiment were held overnight at 5°C and reanalyzed the following day to determine whether a positive signal could be recovered from the epoetin alfa beads. These data are depicted in Fig. 5. On average, 12 MFI units were lost after this extended incubation (range, 3 to 26 MFI units). Even though signal from the spiked samples from donors 16, 18, and 19 was diminished to the level of analytical cutoff (3.3 fluorescence intensity units), all spiked samples scored positive in this scenario. In practice, it is clear that samples should not be held for extended periods of time prior to analysis unless the decrease in MFI is studied and accounted for in the validation stages of assay development. For our purposes here, the minimal loss of signal that resulted from holding the samples is one indication that the analytical platform is robust.
FIG. 5.
Degradation of signal in specimens held for an extended time period. To demonstrate stability of positive signals in this assay, the specimens from Fig. 4 were held overnight at 5°C and analyzed the following day. The drop-off in signal (3 to 26 MFI units; 12 MFI units on average) was consistent between samples and would not impact identification of positive specimens, provided that their relative antibody concentration was >50 ng/ml.
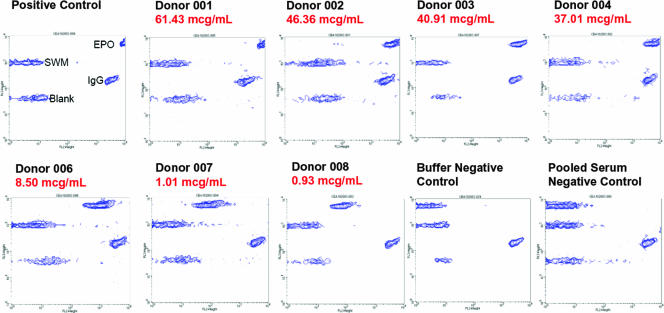
Perhaps the most important component of the validation exercise was provided by testing serum from seven patients with prior diagnosis of antibody-mediated PRCA (Fig. 6) (5). In the cytometric bead assay described here, epoetin alfa-specific antibodies were readily detected in 7 of 7 (100%) of these patients, with a concordance between the magnitude of signal observed in the cytometric bead immunoassay versus prior published data collected with a Biacore immunoassay that serves as a gold standard in our laboratory (22). Relative to the interassay variability calculations provided in Fig. 3, the antibody concentrations detected in these samples from PRCA patients fell within the range of antibody concentrations with <20% coefficient of variation, which provides for a robust measurement and is well above the detection limit of the assay.
FIG. 6.
Validation of assay performance with seven sera known to be positive for anti-epoetin alfa antibodies. Seven sera from patients treated with EPREX and previously characterized (5, 22) to contain anti-epoetin alfa antibodies were tested in the flow cytometric bead immunoassay to demonstrate that the assay can detect clinically relevant antibody species. For each specimen, the relative antibody concentration, as determined in a Biacore immunoassay (22), is indicated in red. The specimens are rank ordered, from the highest antibody concentration to the lowest. Visual inspection of the fluorescence intensity of signal from the epoetin alfa (EPO) bead shows that the flow cytometric bead immunoassay is in concordance with the relative antibody concentration estimated by the Biacore method.
DISCUSSION
Detection of antibodies against therapeutic proteins is an important responsibility of the biopharmaceutical industry. Our laboratory develops, validates, and implements a variety of immunoassays and bioassays during preclinical and clinical development to limit the risks for patients in our clinical trials while also recognizing that pharmacovigilance in the postmarketing stage is essential. Even a low incidence of pathological antibodies can present the industry with safety concerns, especially when there may be safer therapeutic alternatives to be considered. Unfortunately, the immune response to protein-based therapeutics is somewhat unpredictable and, once triggered, plasma cells can rapidly expand with a concomitant rise in serum antibody concentration. The long half-life of antigen-specific plasma cells and the antibodies they produce makes prompt detection of pathological antibodies very important to the individual patient.
For these reasons, our laboratory remains committed to continued evaluation of immunoassay platforms that meet the need to monitor immunogenicity profiles of therapeutic proteins. The cytometric bead immunoassay presented here did not appear to suffer from background noise that is sometimes associated with some serum-based immunoassays (personal observation), and this likely reflects the use of 1% serum in the assay. The low serum concentration required for the assay is made possible because of the recognized sensitivity of flow cytometers and the cytometric bead platform in general. Although our assay may be considered to be novel in many ways, it is noteworthy that a multiplexed bead assay for anti-granulocyte colony-stimulating factor antibodies has been proposed for use as a diagnostic test (2). Moreover, bead arrays have an established their utility for measurement of autoantibodies in a diagnostic setting (20), creating a favorable precedent for this anti-epoetin alfa cytometric bead array once it is fully developed.
Unlike many multiplexed bead immunoassays for cytokines and other analytes (16), we purposely designed this assay around inclusion of positive and negative controls in each specimen to understand the specificity of antibody binding when present. Donor 12 is a case in point for the need for stringent assay acceptance criteria so that false-positive results are not spuriously generated. In a case such as this, it would be expected that alternative assays could be implemented (by the manufacturer) or that serial sampling could be performed to determine whether a specific rise in relative anti-epoetin alfa IgG concentration could be demonstrated. Perhaps more important than the potential generation of a false-positive result is the chance of a false-negative result resulting from analytical error. Although this possibility can never be absolutely excluded from any assay, the inclusion of a positive control bead coupled to IgG can be used to ensure that the fluorochrome-conjugated anti-IgG (detection reagent) was not degraded and was capable of detecting the anti-epoetin alfa antibody in every sample that is analyzed. Moreover, the reproducibility of signal from the positive and negative control beads can be used to establish the dynamic range of the assay, which can be monitored as the assay is run in real time to safeguard against drift in assay performance as a function of time.
Development of antibody-mediated PRCA is an important but infrequent event (18), and we relied on clinical samples obtained through collaboration from Nicole Casadevall, who is a recognized expert in this field and is often consulted as an independent reference under such circumstances. Samples previously characterized to be positive for neutralizing antibodies in her laboratory (5) and further characterized by a Biacore immunoassay, a bridging enzyme-linked immunosorbent assay, a radioimmunoprecipitation assay, and a bioassay in our group (22) were evaluated in the cytometric bead immunoassay, and concordance was observed. Although the sample size is limited to n = 7 in this effort, we view this as a good indicator of assay performance overall; in our prior efforts the inability of the bridging enzyme-linked immunosorbent assay to detect anti-epoetin antibodies in some cases of PRCA (22) was demonstrated even with these small numbers of samples. In future studies of the cytometric bead array, it will be important to perform assessments in population-based studies of patients without PRCA; our prior experience with radioimmunoprecipitation assays and Biacore-based assessments to date indicates that only a minority of persons will have detectable antibodies, that these antibodies will not be associated with clinical pathology or have neutralizing potential in vitro, and that their concentrations will fall in the low nanogram-per-milliliter range (J. Ferbas et al., unpublished data).
We have previously reported on the superiority of the Biacore immunoassay for clinical development programs because of the ease by which all antibody isotypes and subclasses can be detected (23). This is reflected in the readout of the Biacore method as a simple increase in mass on the sensorchip surface, whereas the cytometric bead immunoassay relies upon isotype or subclass-specific fluorochrome-conjugated antibodies for the generation of signal. To date, we have demonstrated that the use of a secondary detection reagent does not have obvious detrimental impact on the assay, and we have successfully performed validation exercises with human samples of known clinical relevance. Although these efforts are not in themselves adequate to conclude that the flow cytometric immunoassay could replace the Biacore platform, they do support the decision to place additional effort toward fully testing and further designing the assay for potential clinical application, and such efforts are under way in our laboratory. Moreover, it is hoped that the proof-of-concept that is offered by the experiments presented here will motivate laboratories to consider the cytometric bead array as a viable alternative platform to monitor patients for immunogenicity events whether they receive erythropoietic stimulating agents or other recombinant protein therapeutics in the clinical arena.
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Bennett, C. L., D. Cournoyer, K. R. Carson, J. Rossert, S. Luminari, A. M. Evens, F. Locatelli, S. M. Belknap, J. M. McKoy, E. A. Lyons, B. Kim, R. Sharma, S. Costello, E. B. Toffelmire, G. A. Wells, H. A. Messner, P. R. Yarnold, S. M. Trifilio, D. W. Raisch, T. M. Kuzel, A. Nissenson, L. C. Lim, M. S. Tallman, and N. Casadevall. 2005. Long-term outcome of individuals with pure red cell aplasia and antierythropoietin antibodies in patients treated with recombinant epoetin: a follow-up report from the Research on Adverse Drug Events and Reports (RADAR) project. Blood 1063343-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonfield, T. L., N. John, B. P. Barna, M. S. Kavuru, M. J. Thomassen, and B. Yen-Lieberman. 2005. Multiplexed particle-based anti-granulocyte macrophage colony stimulating factor assay used as pulmonary diagnostic test. Clin. Diagn. Lab. Immunol. 12821-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boven, K., J. Knight, F. Bader, J. Rossert, K. U. Eckardt, and N. Casadevall. 2005. Epoetin-associated pure red cell aplasia in patients with chronic kidney disease: solving the mystery. Nephrol. Dialysis Transplant. 20iii33-iii40. [DOI] [PubMed] [Google Scholar]
- 4.Boven, K., S. Stryker, J. Knight, A. Thomas, M. van Regenmortel, D. M. Kemeny, D. Power, J. Rossert, and N. Casadevall. 2005. The increased incidence of pure red cell aplasia with an Eprex formulation in uncoated rubber stopper syringes. Kidney Int. 672346-2353. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall, N., J. Nataf, B. Viron, A. Kolta, J. J. Kiladjian, P. Martin-Dupont, P. Michaud, T. Papo, V. Ugo, I. Teyssandier, B. Varet, and P. Mayeux. 2002. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med. 346469-475. [DOI] [PubMed] [Google Scholar]
- 6.Casadevall, N., and J. Rossert. 2005. Importance of biologic follow-ons: experience with EPO. Best Pract. Res. Clin. Hematol. 18381-387. [DOI] [PubMed] [Google Scholar]
- 7.Dias, D., J. Van Doren, S. Schlottmann, S. Kelly, D. Puchalski, W. Ruiz, P. Boerckel, J. Kessler, J. M. Antonello, T. Green, M. Brown, J. Smith, N. Chirmule, E. Barr, K. U. Jansen, and M. T. Esser. 2005. Optimization and validation of a multiplexed Luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin. Diagn. Lab. Immunol. 12959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dighiero, G., B. Guilbert, and S. Avrameas. 1982. Naturally occurring antibodies against nine common antigens in humans sera. II. High incidence of monoclonal Ig exhibiting antibody activity against actin and tubulin and sharing antibody specificities with natural antibodies. J. Immunol. 1282788-2792. [PubMed] [Google Scholar]
- 9.Guilbert, B., G. Dighiero, and S. Avrameas. 1982. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation, and characterization. J. Immunol. 1282779-2787. [PubMed] [Google Scholar]
- 10.Hashida, S., M. Imagawa, S. Inoue, K. H. Ruan, and E. Ishikawa. 1984. More useful maleimide compounds for the conjugation of Fab′ to horseradish peroxidase through thiol groups in the hinge. J. Appl. Biochem. 656-63. [PubMed] [Google Scholar]
- 11.Koren, E., L. A. Zuckerman, and A. R. Mire-Sluis. 2002. Immune responses to therapeutic proteins in humans: clinical significance, assessment and prediction. Curr. Pharm. Biotechnol. 3349-360. [DOI] [PubMed] [Google Scholar]
- 12.Locatelli, F., P. Aljama, P. Barany, B. Canaud, F. Carrera, K. U. Eckardt, I. C. MacDougall, A. Macleod, W. H. Horl, A. Wiecek, and S. Cameron. 2004. Erythropoiesis-stimulating agents and antibody-mediated pure red-cell aplasia: here are we now and where do we go from here? Nephrol. Dialysis Transplant. 19288-293. [DOI] [PubMed] [Google Scholar]
- 13.Louet, S. 2003. Lessons from Eprex for biogeneric firms. Nat. Biotechnol. 21956-957. [DOI] [PubMed] [Google Scholar]
- 14.Mason, S., S. La, D. Mytych, S. J. Swanson, and J. Ferbas. 2003. Validation of the BIACORE 3000 platform for detection of antibodies against erythropoietic agents in human serum samples. Curr. Med. Res. Opin. 19651-659. [DOI] [PubMed] [Google Scholar]
- 15.Mire-Sluis, A. R., Y. C. Barrett, V. Devanarayan, E. Koren, H. Liu, M. Maia, T. Parish, G. Scott, G. Shankar, and E. Shores. 2004. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J. Immunol. Methods 2891-16. [DOI] [PubMed] [Google Scholar]
- 16.Morgan, E., R. Varro, H. Sepulveda, J. A. Ember, J. Apgar, J. Wilson, L. Lowe, R. Chen, L. Shivraj, A. Agadir, R. Campos, D. Ernst, and A. Gaur. 2004. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin. Immunol. 110252-266. [DOI] [PubMed] [Google Scholar]
- 17.Porter, S. 2001. Human immune response to recombinant human proteins. J. Pharm. Sci. 901-11. [DOI] [PubMed] [Google Scholar]
- 18.Rossert, J. 2005. Erythropoietin-induced, antibody mediated pure red cell aplasia. Eur. J. Clin. Investig. 35(Suppl. 3)95-99. [DOI] [PubMed] [Google Scholar]
- 19.Schellekens, H., and W. Jiskoot. 2006. Eprex-associated pure red cell aplasia and leachates. Nat. Biotechnol. 24613-614. [DOI] [PubMed] [Google Scholar]
- 20.Shovman, O., B. Gilburd, O. Barzilai, E. Shinar, B. Larida, G. Zandma-Goddard, S. R. Binder, and Y. Shoenfeld. 2005. Evaluation of the BioPlex 2200 ANA screen: analysis of 510 healthy subjects: incidence of natural/predictive autoantibodies. Ann. N. Y. Acad. Sci. 1050380-388. [DOI] [PubMed] [Google Scholar]
- 21.Suzanne, H., J. Wim, J. A. C. Daan, and S. Huub. 2006. Reaction to the paper: interaction of polysorbate 80 with erythropoietin: a case study in protein surfactant Interactions. Pharm. Res. V 23641-642. [DOI] [PubMed] [Google Scholar]
- 22.Swanson, S. J., J. Ferbas, P. Mayeux, and N. Casadevall. 2004. Evaluation of methods to detect and characterize antibodies against recombinant human erythropoietin. Nephron Clin. Pract. 96c88-c95. [DOI] [PubMed] [Google Scholar]
- 23.Swanson, S. J., D. Mytych, and J. Ferbas. 2002. Use of biosensors to monitor the immune response. Dev. Biol. 10971-78. [PubMed] [Google Scholar]
- 24.Thorpe, R., and S. J. Swanson. 2005. Assays for detecting and diagnosing antibody-mediated pure red cell aplasia (PRCA): an assessment of available procedures. Nephrol. Dialysis Transplant. 20iv16-iv22. [DOI] [PubMed] [Google Scholar]
- 25.Twining, S. S., H. F. Lehmann, and M. Z. Atassi. 1980. The antibody response to myoglobin is independent of the immunized species: analysis in terms of replacements in the antigenic sites and in environmental residues of the cross-reactions of fifteen myoglobins with sperm-whale myoglobin antisera raised in different species. Biochem. J. 191681-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei, X., S. J. Swanson, and S. Gupta. 2004. Development and validation of a cell-based bioassay for the detection of neutralizing antibodies against recombinant human erythropoietin in clinical studies. J. Immunol. Methods 293115-126. [DOI] [PubMed] [Google Scholar]