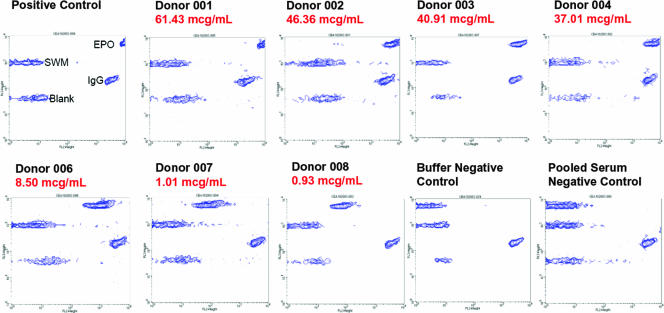
FIG. 6.
Validation of assay performance with seven sera known to be positive for anti-epoetin alfa antibodies. Seven sera from patients treated with EPREX and previously characterized (5, 22) to contain anti-epoetin alfa antibodies were tested in the flow cytometric bead immunoassay to demonstrate that the assay can detect clinically relevant antibody species. For each specimen, the relative antibody concentration, as determined in a Biacore immunoassay (22), is indicated in red. The specimens are rank ordered, from the highest antibody concentration to the lowest. Visual inspection of the fluorescence intensity of signal from the epoetin alfa (EPO) bead shows that the flow cytometric bead immunoassay is in concordance with the relative antibody concentration estimated by the Biacore method.