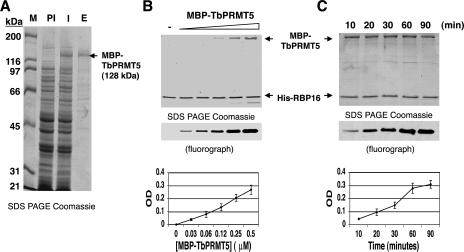
FIG. 2.
Recombinant MBP-TbPRMT5 exhibits time- and concentration-dependent in vitro methyltransferase activity. (A) Coomassie-stained 10% SDS-PAGE gel showing the partial purification of recombinant TbPRMT5. TbPRMT5 was cloned and expressed as an N-terminal MBP fusion protein and partially purified by affinity chromatography over amylose resin. M, markers; PI, preinduced; I, induced; E, eluate from amylose column. (B) Recombinant MBP-TbPRMT5 exhibits concentration-dependent in vitro methyltransferase activity. In vitro methylation assays were performed in triplicate for 1 h at 36°C in the presence of 1 μM [methyl-3H]AdoMet, 5 μM His-RBP16, and increasing concentrations of MBP-TbPRMT5. The reactions were stopped with the addition of SDS sample buffer, resolved by 15% polyacrylamide SDS-PAGE, and analyzed by fluorography. Bands were quantified using Bio-Rad Quantity One imaging software. The background OD was subtracted and set to 0. The graph shows the mean ± standard deviation of triplicate experiments. A representative fluorograph and corresponding Coomassie-stained gel are shown above the graph. The positions of MBP-TbPRMT5 and His-RBP16 in the Coomassie-stained gel are indicated with arrows. (C) Time course of MBP-TbPRMT5 in vitro methyltransferase activity. In vitro methyltransferase assays were performed in triplicate at 36°C in the presence of 1 μM [methyl-3H]AdoMet, 3 μM His-RBP16, and 0.3 μM MBP-TbPRMT5. Aliquots were taken at the indicated time points, and the reactions were stopped with the addition of SDS sample buffer. The reactions were analyzed as described for panel B, except baseline OD values were normalized to the minimal value of triplicate experiments. Labels as in panel B.