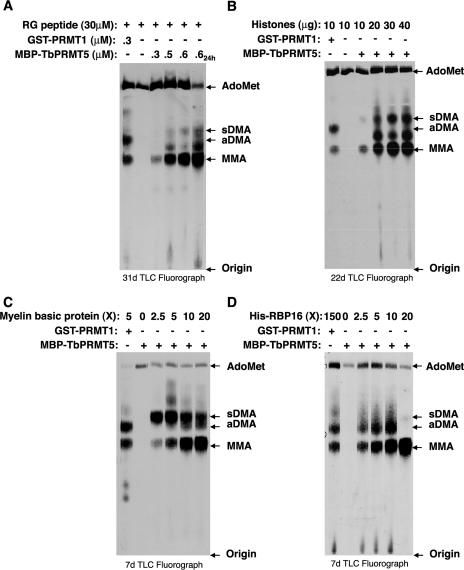
FIG. 4.
Recombinant MBP-TbPRMT5 catalyzes the formation of monomethylarginine and symmetric dimethylarginine in a substrate- and concentration-dependent manner. (A) Amino acid analysis of an RG peptide methylated in vitro by recombinant MBP-TbPRMT5. In vitro methylation assays were performed at 36°C for 1 or 24 h (far-right lane) in the presence of 1 μM [methyl-3H]AdoMet, 30 μM RG peptide substrate, and either 0.3 μM GST-PRMT1 or the indicated concentrations of MBP-TbPRMT5. Samples were acid hydrolyzed to free amino acids and resolved by TLC alongside 30 nmol of the indicated amino acid standards shown to the right, as described in Materials and Methods. (B) Amino acid analysis of methylated calf thymus core histones. Assays were performed for 16 h at 36°C in the presence of 1 μM [methyl-3H]AdoMet, 10 to 40 μg core histone substrate, and either 0.07 μM GST-PRMT1 or 0.13 μM MBP-TbPRMT5. The reactions were stopped and analyzed as described for panel A. (C) Amino acid analysis of methylated myelin basic protein. Assays were performed for 16 h at 36°C in the presence of 1 μM [methyl-3H]AdoMet, the indicated molar excess (X) of myelin basic protein substrate, and either 2.2 μM GST-PRMT1 or 0.5 μM MBP-TbPRMT5. The reactions were stopped and analyzed as described for panel A. (D) Amino acid analysis of methylated His-RBP16. Assays were performed for 16 h at 36°C in the presence of 1 μM [methyl-3H]AdoMet, the indicated molar excess (X) of His-RBP16 substrate, and either 0.05 μM GST-PRMT1 or 0.7 μM MBP-TbPRMT5. The reactions were stopped and analyzed as described for panel A.